|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110852 |
|---|
|
Identification |
|---|
| Name: |
chorismate |
|---|
| Description: | Chorismic acid, more commonly known as its anionic form chorismate, is an important biochemical intermediate in plants and microorganisms. It is a precursor for the aromatic amino acids phenylalanine and tyrosine,indole, indole derivatives and tryptophan,2,3-dihydroxybenzoic acid (DHB) used for enterobactin biosynthesis,the plant hormone salicylic acid and many alkaloids and other aromatic metabolites. -- Wikipedia. |
|---|
|
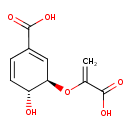
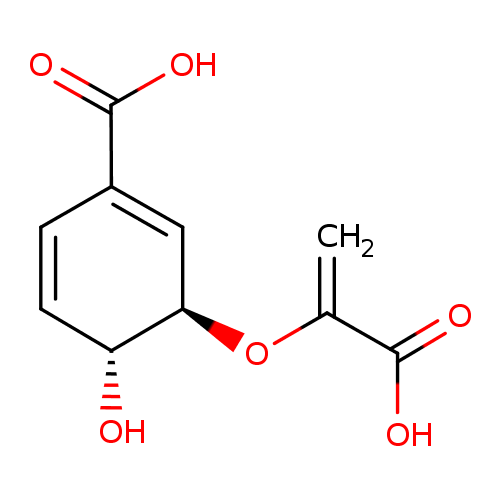
Structure |
|
|---|
| Synonyms: | |
|---|
|
Chemical Formula: |
C10H8O6
|
|---|
| Average Molecular Weight: |
224.17 |
|---|
| Monoisotopic Molecular
Weight: |
226.0477380536 |
|---|
| InChI Key: |
WTFXTQVDAKGDEY-HTQZYQBOSA-L |
|---|
| InChI: |
InChI=1S/C10H10O6/c1-5(9(12)13)16-8-4-6(10(14)15)2-3-7(8)11/h2-4,7-8,11H,1H2,(H,12,13)(H,14,15)/p-2/t7-,8-/m1/s1 |
|---|
| CAS
number: |
55508-12-8 |
|---|
| IUPAC Name: | (3R,4R)-3-[(1-carboxylatoethenyl)oxy]-4-hydroxycyclohexa-1,5-diene-1-carboxylate |
|---|
|
Traditional IUPAC Name: |
chorismic acid |
|---|
| SMILES: | C=C(C(=O)[O-])OC1(C(O)C=CC(C([O-])=O)=C1) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as dicarboxylic acids and derivatives. These are organic compounds containing exactly two carboxylic acid groups. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Carboxylic acids and derivatives |
|---|
|
Direct Parent |
Dicarboxylic acids and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Dicarboxylic acid or derivatives
- Secondary alcohol
- Carboxylic acid
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic homomonocyclic compounds |
|---|
| External Descriptors |
- 5-[(1-carboxyethenyl)oxy]-6-hydroxycyclohexa-1,3-diene-1-carboxylic acid (CHEBI:17333 )
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
140 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 140 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|