|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110845 |
|---|
|
Identification |
|---|
| Name: |
β-nicotinamide D-ribonucleotide |
|---|
| Description: | Nicotinamide ribotide (NMN) is an important intermediate metabolite in the nicotinate and nicotinamide metabolism pathway. Mammals predominantly use nicotinamide rather than nicotinic acid as a precursor for NAD biosynthesis. Instead of the deamidation to nicotinic acid, nicotinamide is directly converted to NMN by nicotinamide phosphoribosyltransferase (NAMPT, EC 2.4.2.12). The enzyme nicotinamide mononucleotide adenylyltransferase (NMNAT, EC 2.7.7.1), which is a member of the nucleotidyltransferase alpha/beta-phosphodiesterase superfamily, catalyzes the reaction NMN + ATP <=> Nicotinamide adenine dinucleotide (NAD) + PPi, representing the final step in the biosynthesis of NAD. NAD is a molecule that plays a fundamental role as a cofactor in cellular redox reactions. Thus NMN is an important metabolite for the maintenance of normal NAD biosynthesis. Circulating NMN levels may play an important role in regulating cell function in physiological and pathophysiological conditions. (PMID: 15078171 , 17983582 ). |
|---|
|
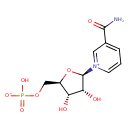
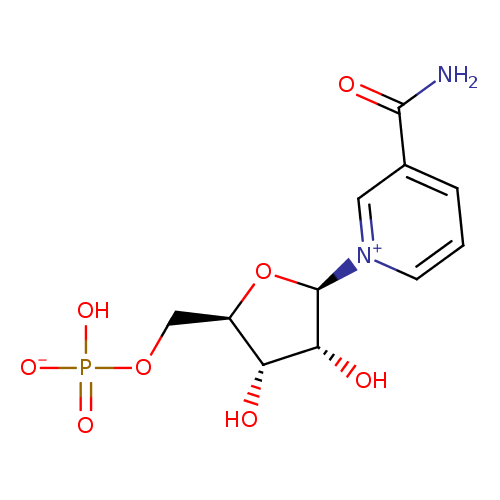
Structure |
|
|---|
| Synonyms: | -
β-nicotinamide mononucleotide
-
β-nicotinamide ribonucleotide
-
β-nicotinamide nucleotide
-
NMN
|
|---|
|
Chemical Formula: |
C11H14N2O8P
|
|---|
| Average Molecular Weight: |
333.21 |
|---|
| Monoisotopic Molecular
Weight: |
335.0644270108 |
|---|
| InChI Key: |
DAYLJWODMCOQEW-TURQNECASA-M |
|---|
| InChI: |
InChI=1S/C11H15N2O8P/c12-10(16)6-2-1-3-13(4-6)11-9(15)8(14)7(21-11)5-20-22(17,18)19/h1-4,7-9,11,14-15H,5H2,(H3-,12,16,17,18,19)/p-1/t7-,8-,9-,11-/m1/s1 |
|---|
| CAS
number: |
1094-61-7 |
|---|
| IUPAC Name: | 3-carbamoyl-1-(5-O-phosphonato-β-D-ribofuranosyl)pyridinium |
|---|
|
Traditional IUPAC Name: |
nmn zwitterion |
|---|
| SMILES: | C(OP([O-])(=O)[O-])C1(C(O)C(O)C(O1)[N+]2(C=CC=C(C(=O)N)C=2)) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as nicotinamide nucleotides. These are pyridine nucleotides, in which the pyridine base is nicotinamide or a derivative thereof. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Nucleosides, nucleotides, and analogues |
|---|
| Sub Class | Pyridine nucleotides |
|---|
|
Direct Parent |
Nicotinamide nucleotides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Nicotinamide-nucleotide
- Pentose phosphate
- Pentose-5-phosphate
- Glycosyl compound
- N-glycosyl compound
- Monosaccharide phosphate
- Pentose monosaccharide
- Nicotinamide
- Pyridine carboxylic acid or derivatives
- Monosaccharide
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Pyridine
- Pyridinium
- Alkyl phosphate
- Vinylogous amide
- Oxolane
- Heteroaromatic compound
- Secondary alcohol
- Carboxamide group
- 1,2-diol
- Primary carboxylic acid amide
- Carboxylic acid derivative
- Organoheterocyclic compound
- Oxacycle
- Azacycle
- Organooxygen compound
- Organonitrogen compound
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Organic zwitterion
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- Nicotinate and Nicotinamide Metabolism pae00760
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS | Not Available |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-00di-1931000000-5aa0e5ba467b6bdbb57f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-00dj-6920000000-4720b1b8ab108dc1b499 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-00mk-8903000000-042bd96d57e63adafe44 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive | splash10-00di-3900000000-013c2f02e48342e61b5e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,1H] 2D NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Ohdoi C, Nyhan WL, Kuhara T: Chemical diagnosis of Lesch-Nyhan syndrome using gas chromatography-mass spectrometry detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2003 Jul 15;792(1):123-30. [12829005 ]
- Schuberth J: Volatile compounds detected in blood of drunk drivers by headspace/capillary gas chromatography/ion trap mass spectrometry. Biol Mass Spectrom. 1991 Nov;20(11):699-702. [1799580 ]
- Emanuelli M, Raffaelli N, Amici A, Balducci E, Natalini P, Ruggieri S, Magni G: The antitumor drug, 1,3-bis(2-chloroethyl)-1-nitroso-urea, inactivates human nicotinamide mononucleotide adenylyltransferase. Biochem Pharmacol. 1995 Feb 14;49(4):575-9. [7872964 ]
- Moriya F, Hashimoto Y: Postmortem production of ethanol and n-propanol in the brain of drowned persons. Am J Forensic Med Pathol. 2004 Jun;25(2):131-3. [15166764 ]
- Magni G, Amici A, Emanuelli M, Orsomando G, Raffaelli N, Ruggieri S: Structure and function of nicotinamide mononucleotide adenylyltransferase. Curr Med Chem. 2004 Apr;11(7):873-85. [15078171 ]
- Revollo JR, Korner A, Mills KF, Satoh A, Wang T, Garten A, Dasgupta B, Sasaki Y, Wolberger C, Townsend RR, Milbrandt J, Kiess W, Imai S: Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007 Nov;6(5):363-75. [17983582 ]
|
|---|
| Synthesis Reference: |
Liu, Rihe; Visscher, Johannes. A novel preparation of nicotinamide mononucleotide. Nucleosides & Nucleotides (1994), 13(5), 1215-16. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|