|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110834 |
|---|
|
Identification |
|---|
| Name: |
thiosulfate |
|---|
| Description: | Thiosulfate occurs naturally in hot springs and geysers, and is produced by certain biochemical processes. In the body, thiosulfate converts small amounts of cyanide ion into harmless products and plays a role in the biosynthesis of cysteine, a sulfur-containing amino acid that locks proteins into their correct three-dimensional shapes. Thiosulfate is not found in large quantities in nature. Solutions of thiosulfate break down into sulfur, sulfites, and sulfates when exposed to acids, light, metal ions, and bacteria. Thiosulfate is sometimes used as an antidote for cyanide poisoning. It reacts with cyanide to produce sulfite and thiocyanate ions: CN- + S2O32- SCN- + SO32- This reaction is catalyzed by an enzyme produced by cell mitochondria to neutralize small quantities of ingested cyanide (which occurs naturally in cassava root, lima beans, and almonds!). Thiosulfate is an intermediate in several biochemical pathways, including the synthesis of L-cysteine. Thiosulfate is manufactured by some cells by oxidation of elemental sulfur and by degradation of L-cysteine. Use: Photography (fixing agent to dissolve unchanged silver salts from exposed negatives), chrome tanning, removing chlorine in bleaching and papermaking, extraction of silver from its ores, dechlorination of water, mordant, reagent, bleaching, reducing agent in chrome dyeing, sequestrant in salt (up to 0.1%), antidote for cyanide poisoning. (Hawley's Condensed Chemical Dictionary) Source/Synthesis: Synthesis by dehydration of the pentahydrate at 105 degree. Alternatively formed by reaction of S2Cl2 with Na2O2 or by reduction of Na2S2O4 with sodium amalgam Use/Importance: Commercially available Biological Use/Importance: Cyanide antidote often administered with other antidotes, antifungal agent (ChemNetBase) Sodium thiosulfate is a common analytical reagent used in iodometric titration to analyze chlorine, bromine, and sulfide. Other uses are in bleaching paper pulp, bleaching straw, ivory, and bones, for removing chlorine from solutions, silver extraction from its ores, a mordant in dyeing and printing textiles, and as an antidote to cyanide poisoning. Another major application is in photography, where it is used as a fixer to dissolve unchanged silver salts from exposed negatives. (Handbook of Inorganic Chemicals). |
|---|
|
Structure |
|
|---|
| Synonyms: | |
|---|
|
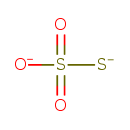
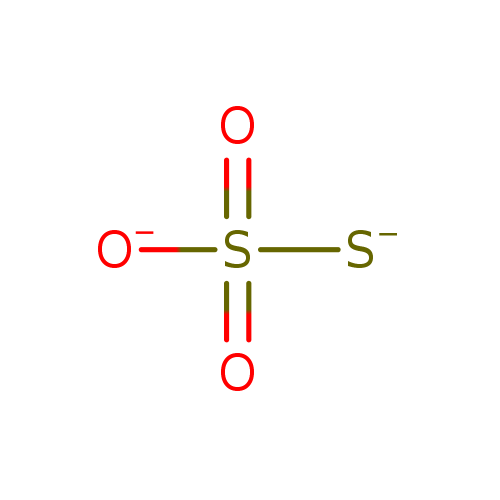
Chemical Formula: |
HO3S2
|
|---|
| Average Molecular Weight: |
113.13 |
|---|
| Monoisotopic Molecular
Weight: |
113.9445353105 |
|---|
| InChI Key: |
DHCDFWKWKRSZHF-UHFFFAOYSA-M |
|---|
| InChI: |
InChI=1S/H2O3S2/c1-5(2,3)4/h(H2,1,2,3,4)/p-1 |
|---|
| CAS
number: |
14383-50-7 |
|---|
| IUPAC Name: | hydroxidodioxidosulfidosulfate(1−) |
|---|
|
Traditional IUPAC Name: |
thiosulphate |
|---|
| SMILES: | O=S(=O)([O-])S |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as non-metal thiosulfates. These are inorganic non-metallic compounds containing a thiosulfate as its largest oxoanion. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Inorganic compounds |
|---|
|
Class |
Homogeneous non-metal compounds |
|---|
| Sub Class | Non-metal oxoanionic compounds |
|---|
|
Direct Parent |
Non-metal thiosulfates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Non-metal thiosulfate
- Inorganic oxide
- Inorganic sulfide
|
|---|
| Molecular Framework |
Not Available |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
48 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 48 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 861 mg/mL | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Willis CL, Cummings JH, Neale G, Gibson GR: Nutritional aspects of dissimilatory sulfate reduction in the human large intestine. Curr Microbiol. 1997 Nov;35(5):294-8. [9462959 ]
- Chatterjee BD, De PK, Sen T: Sucrose teepol tellurite agar: a new selective indicator medium for isolation of Vibrio species. J Infect Dis. 1977 Apr;135(4):654-8. [856920 ]
- Rikimaru T, Kondo M, Kajimura K, Hashimoto K, Oyamada K, Sagawa K, Tanoue S, Oizumi K: Bactericidal activities of commonly used antiseptics against multidrug-resistant mycobacterium tuberculosis. Dermatology. 2002;204 Suppl 1:15-20. [12011515 ]
- Maddocks JL, MacLachlan J: Application of new fluorescent thiol reagent to diagnosis of homocystinuria. Lancet. 1991 Oct 26;338(8774):1043-4. [1681358 ]
- Ivankovich AD, Braverman B, Stephens TS, Shulman M, Heyman HJ: Sodium thiosulfate disposition in humans: relation to sodium nitroprusside toxicity. Anesthesiology. 1983 Jan;58(1):11-7. [6600205 ]
- Kage S, Nagata T, Kudo K: Determination of thiosulfate in body fluids by GC and GC/MS. J Anal Toxicol. 1991 May-Jun;15(3):148-50. [1943059 ]
- Rikimaru T, Kondo M, Kondo S, Oizumi K: Bactericidal activities of povidone-iodine against Mycobacterium. Dermatology. 1997;195 Suppl 2:104-6. [9403266 ]
- Zewert TE, Pliquett UF, Vanbever R, Langer R, Weaver JC: Creation of transdermal pathways for macromolecule transport by skin electroporation and a low toxicity, pathway-enlarging molecule. Bioelectrochem Bioenerg. 1999 Oct;49(1):11-20. [10619443 ]
- Freyberg RH, Block WD, Levey S: METABOLISM, TOXICITY AND MANNER OF ACTION OF GOLD COMPOUNDS USED IN THE TREATMENT OF ARTHRITIS. I. HUMAN PLASMA AND SYNOVIAL FLUID CONCENTRATION AND URINARY EXCRETION OF GOLD DURING AND FOLLOWING TREATMENT WITH GOLD SODIUM THIOMALATE, GOLD SODIUM THIOSULFATE, AND COLLOIDAL GOLD SULFIDE. J Clin Invest. 1941 Jul;20(4):401-12. [16694848 ]
- Westerlund J, Pudek M, Schreiber WE: A rapid and accurate spectrofluorometric method for quantification and screening of urinary porphyrins. Clin Chem. 1988 Feb;34(2):345-51. [3342508 ]
- Yatzidis H: Gestational urinary hyperthiosulfaturia protects hypercalciuric normal pregnant women from nephrolithiasis. Int Urol Nephrol. 2004;36(3):445-9. [15783122 ]
- Rennels MB, Levine MM, Daya V, Angle P, Young C: Selective vs. nonselective media and direct plating vs. enrichment technique in isolation of Vibrio cholerae: recommendations for clinical laboratories. J Infect Dis. 1980 Sep;142(3):328-31. [7003031 ]
|
|---|
| Synthesis Reference: |
Serikova, E. A.; Racheva, I. V. Method for producing sodium thiosulfate. U.S.S.R. (1986), CODEN: URXXAF SU 1279954 A1 19861230 Patent written in Russian. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|