|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110824 |
|---|
|
Identification |
|---|
| Name: |
4-maleyl-acetoacetate |
|---|
| Description: | 4-Maleylacetoacetate is an intermediate in the metabolism of tyrosine. Homogentisate 1,2-dioxygenase is the enzyme, which catalyzes the conversion of homogentisate to 4-maleylacetoacetate. Homogentisate 1,2-dioxygenase or HGD is involved in the catabolism of aromatic rings, more specifically in the break down of the amino acids tyrosine and phenylalanine. |
|---|
|
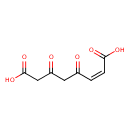
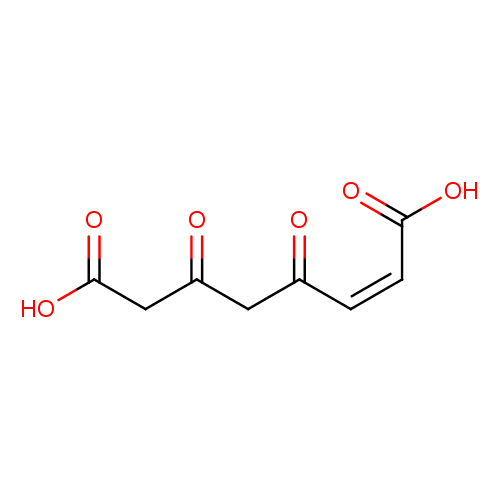
Structure |
|
|---|
| Synonyms: | - 4-Maleylacetoacetate
- 4-Maleylacetoacetic acid
- Maleylacetoacetate
|
|---|
|
Chemical Formula: |
C8H6O6
|
|---|
| Average Molecular Weight: |
198.13 |
|---|
| Monoisotopic Molecular
Weight: |
200.0320879894 |
|---|
| InChI Key: |
GACSIVHAIFQKTC-UPHRSURJSA-L |
|---|
| InChI: |
InChI=1S/C8H8O6/c9-5(1-2-7(11)12)3-6(10)4-8(13)14/h1-2H,3-4H2,(H,11,12)(H,13,14)/p-2/b2-1- |
|---|
| CAS
number: |
5698-52-2 |
|---|
| IUPAC Name: | (2Z)-4,6-dioxooct-2-enedioate |
|---|
|
Traditional IUPAC Name: |
maleylacetoacetic acid |
|---|
| SMILES: | C([O-])(=O)C=CC(=O)CC(=O)CC([O-])=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as medium-chain keto acids and derivatives. These are keto acids with a 6 to 12 carbon atoms long side chain. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Keto acids and derivatives |
|---|
|
Direct Parent |
Medium-chain keto acids and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Medium-chain keto acid
- Beta-keto acid
- 1,3-diketone
- Beta-hydroxy ketone
- Dicarboxylic acid or derivatives
- Unsaturated fatty acid
- 1,3-dicarbonyl compound
- Fatty acyl
- Enone
- Acryloyl-group
- Alpha,beta-unsaturated ketone
- Ketone
- Carboxylic acid
- Carboxylic acid derivative
- Organooxygen compound
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Kvittingen EA, Halvorsen S, Jellum E: Deficient fumarylacetoacetate fumarylhydrolase activity in lymphocytes and fibroblasts from patients with hereditary tyrosinemia. Pediatr Res. 1983 Jul;17(7):541-4. [6622096 ]
- Gray RG, Patrick AD, Preston FE, Whitfield MF: Acute hereditary tyrosinaemia type I: clinical, biochemical and haematological studies in twins. J Inherit Metab Dis. 1981;4(1):37-40. [6785523 ]
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|