|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110823 |
|---|
|
Identification |
|---|
| Name: |
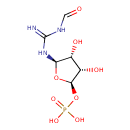
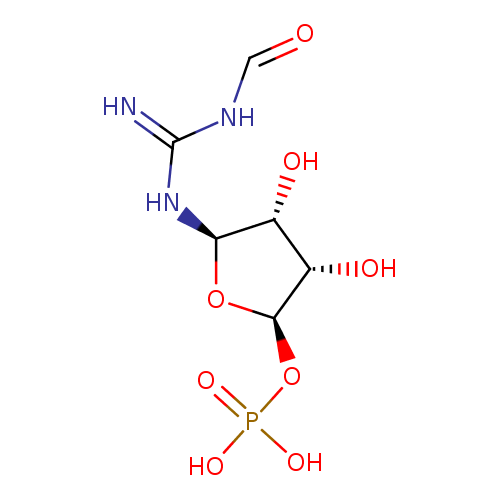
2-(formamido)-N1-(5-phospho-β-D-ribosyl)acetamidine |
|---|
| Description: | 2-(Formamido)-N1-(5-phospho-D-ribosyl)acetamidine is an intermediate in purine metabolism. The enzyme phosphoribosylformylglycinamidine synthase [EC:6.3.5.3] catalyzes the production of this metabolite from N2-formyl-N1-(5-phospho-D-ribosyl)glycinamide. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
5-phosphoribosyl-N-formylglycineamidine
-
5'-phosphoribosyl-N-formyl glycineamidine
-
FGAM
-
5'-phosphoribosylformylglycinamidine
|
|---|
|
Chemical Formula: |
C8H15N3O8P
|
|---|
| Average Molecular Weight: |
312.2 |
|---|
| Monoisotopic Molecular
Weight: |
314.0753260481 |
|---|
| InChI Key: |
PMCOGCVKOAOZQM-XVFCMESISA-M |
|---|
| InChI: |
InChI=1S/C8H16N3O8P/c9-5(1-10-3-12)11-8-7(14)6(13)4(19-8)2-18-20(15,16)17/h3-4,6-8,13-14H,1-2H2,(H2,9,11)(H,10,12)(H2,15,16,17)/p-1/t4-,6-,7-,8-/m1/s1 |
|---|
| CAS
number: |
6157-85-3 |
|---|
| IUPAC Name: | 1-deoxy-1-[2-(formamido)acetimidamido]-D-ribofuranose 5-(dihydrogen phosphate) |
|---|
|
Traditional IUPAC Name: |
[(2R,3S,4R,5R)-3,4-dihydroxy-5-[(formamidomethanimidoyl)amino]oxolan-2-yl]oxyphosphonic acid |
|---|
| SMILES: | C(NC=O)C(=[N+])NC1(C(O)C(O)C(COP([O-])(=O)[O-])O1) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as pentoses. These are monosaccharides in which the carbohydrate moiety contains five carbon atoms. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic oxygen compounds |
|---|
| Sub Class | Organooxygen compounds |
|---|
|
Direct Parent |
Pentoses |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Pentose monosaccharide
- Monoalkyl phosphate
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Oxolane
- 1,2-diol
- Guanidine
- Secondary alcohol
- Oxacycle
- Carboximidamide
- Carboxylic acid derivative
- Organoheterocyclic compound
- Propargyl-type 1,3-dipolar organic compound
- Organic 1,3-dipolar compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organonitrogen compound
- Organic nitrogen compound
- Alcohol
- Carbonyl group
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|