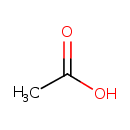
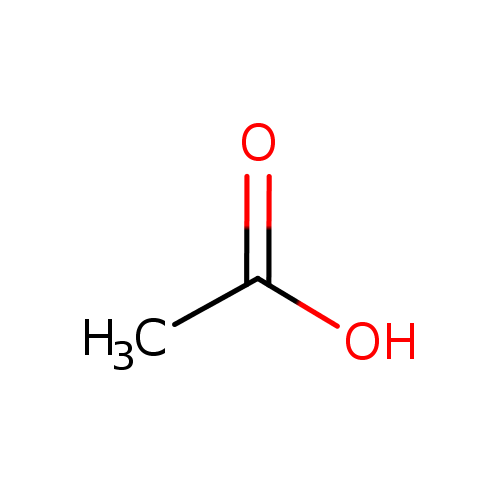
acetate (PAMDB110762)
| Record Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB110762 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | acetate | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | A monocarboxylic acid anion resulting from the removal of a proton from the carboxy group of acetic acid. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C2H3O2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 59.044 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 60.0211293726 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | QTBSBXVTEAMEQO-UHFFFAOYSA-M | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C2H4O2/c1-2(3)4/h1H3,(H,3,4)/p-1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 64-19-7 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | acetate | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | acetic acid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CC([O-])=O | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as carboxylic acids. These are compounds containing a carboxylic acid group with the formula -C(=O)OH. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organic acids and derivatives | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Carboxylic acids and derivatives | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Carboxylic acids | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Carboxylic acids | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Liquid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | 16.6 °C | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Acetaldehyde + NADP+ + Water → acetate + NADPH + Hydrogen ion coenzyme A + acetate + ATP → phosphate + acetyl-CoA + ADP acetate + Carbon dioxide + Hydrogen ion → pyruvate + Water O-Acetylserine + Hydrogen selenide → Selenocysteine + acetate + Hydrogen ion pyruvate + Water + Ubiquinones → Carbon dioxide + Ubiquinols + acetate O-Acetylserine + thiosulfate → Hydrogen ion + S-sulfo-L-cysteine + acetate Water + N-acetylputrescine → putrescine + acetate acetamide + Water → acetate + Ammonium N-acetyl-L-citrulline + Water → Citrulline + acetate Acetaldehyde + Water + NAD+ → acetate + NADH + Hydrogen ion malonate + Hydrogen ion → acetate + Carbon dioxide malonate → acetate O-acetyl-L-homoserine + methanethiol → Hydrogen ion + acetate + L-Methionine Water + N-acetyl-D-galactosamine 6-phosphate → acetate + D-galactosamine 6-phosphate 3-hydroxy-3-(4-methylpent-3-en-1-yl)glutaryl-CoA → 7-methyl-3-oxooct-6-enoyl-CoA + acetate crotonate + acetyl-CoA → crotonyl-CoA + acetate | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Law, David John. Process for the preparation of carboxylic acids and/or derivatives thereof. PCT Int. Appl. (2007), 14pp. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||