|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110755 |
|---|
|
Identification |
|---|
| Name: |
D-threo-isocitrate |
|---|
| Description: | The D-threo-form of isocitrate(3−). |
|---|
|
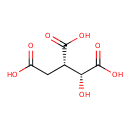
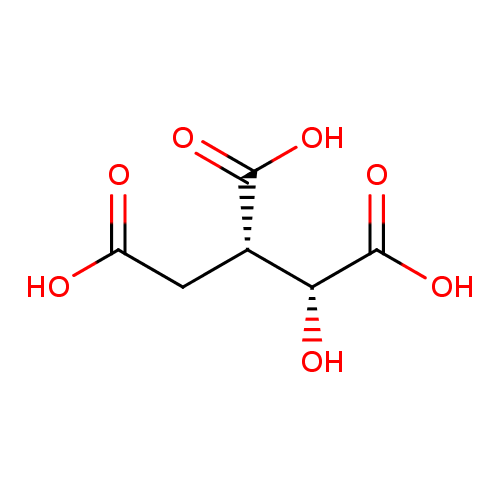
Structure |
|
|---|
| Synonyms: | -
D-threo-isocitrate
-
(1R
- 2S)-1-hydroxypropane-1,2,3-tricarboxylate
-
D-threo-isocitric acid
-
isocitric acid
-
isocitrate
-
threo-Ds-isocitrate
-
I-CIT
-
D-isocitrate
|
|---|
|
Chemical Formula: |
C6H5O7
|
|---|
| Average Molecular Weight: |
189.1 |
|---|
| Monoisotopic Molecular
Weight: |
192.0270026115 |
|---|
| InChI Key: |
ODBLHEXUDAPZAU-ZAFYKAAXSA-K |
|---|
| InChI: |
InChI=1S/C6H8O7/c7-3(8)1-2(5(10)11)4(9)6(12)13/h2,4,9H,1H2,(H,7,8)(H,10,11)(H,12,13)/p-3/t2-,4+/m0/s1 |
|---|
| CAS
number: |
6061-97-8 |
|---|
| IUPAC Name: | (1R,2S)-1-hydroxypropane-1,2,3-tricarboxylate |
|---|
|
Traditional IUPAC Name: |
threo-DS(+)-isocitrate |
|---|
| SMILES: | C(=O)(C(CC([O-])=O)C(O)C([O-])=O)[O-] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as tricarboxylic acids and derivatives. These are carboxylic acids containing exactly three carboxyl groups. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Carboxylic acids and derivatives |
|---|
|
Direct Parent |
Tricarboxylic acids and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Tricarboxylic acid or derivatives
- Beta-hydroxy acid
- Monosaccharide
- Hydroxy acid
- Alpha-hydroxy acid
- Secondary alcohol
- Carboxylic acid
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -3 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS | Not Available |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Negative (Annotated) | splash10-0udi-0900000000-24949c0952ed64de7083 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Negative (Annotated) | splash10-0w29-1900000000-c9bdca817d12cb4b091b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Negative (Annotated) | splash10-014l-9300000000-66e9d659711c1d339f76 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,1H] 2D NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Wong, Chi Huey; Whitesides, George M. Enzyme-catalyzed transhydrogenation between nicotinamide cofactors and its application in organic synthesis. Journal of the American Chemical Society (1982), 104(12), 3542-4. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|