|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110737 |
|---|
|
Identification |
|---|
| Name: |
gentisate |
|---|
| Description: | A dihydroxybenzoate that is the conjugate base of 2,5-dihydroxybenzoic acid; major species at pH 7.3. |
|---|
|
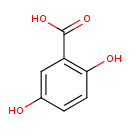
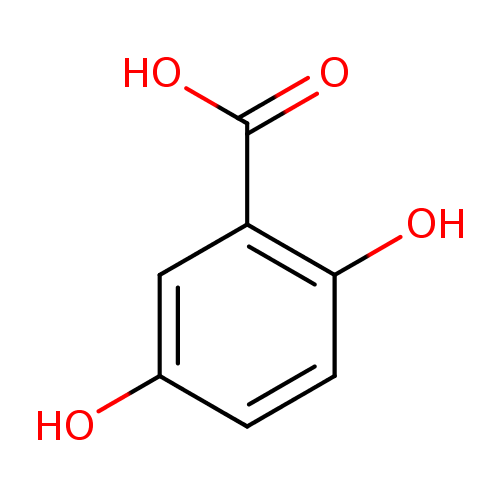
Structure |
|
|---|
| Synonyms: | -
gentisic acid
-
hydroquinonecarboxylic acid
-
2,5-dihydroxybenzoate
-
2,5-DHBA
-
2,5-dihydroxybenzoic acid
-
2,5-dioxybenzoic acid
-
3,6-dihydroxybenzoic acid
-
5-hydroxysalicylic acid
-
5-hydroxysalicylate
|
|---|
|
Chemical Formula: |
C7H5O4
|
|---|
| Average Molecular Weight: |
153.11 |
|---|
| Monoisotopic Molecular
Weight: |
154.026608681 |
|---|
| InChI Key: |
WXTMDXOMEHJXQO-UHFFFAOYSA-M |
|---|
| InChI: |
InChI=1S/C7H6O4/c8-4-1-2-6(9)5(3-4)7(10)11/h1-3,8-9H,(H,10,11)/p-1 |
|---|
| CAS
number: |
490-79-9 |
|---|
| IUPAC Name: | 2,5-dihydroxybenzoate |
|---|
|
Traditional IUPAC Name: |
2,5-dihydroxybenzoic acid |
|---|
| SMILES: | C(=O)([O-])C1(=C(O)C=CC(O)=C1) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as hydroxybenzoic acid derivatives. These are compounds containing a hydroxybenzoic acid (or a derivative), which is a benzene ring bearing a carboxyl and a hydroxyl groups. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
|
Class |
Benzene and substituted derivatives |
|---|
| Sub Class | Benzoic acids and derivatives |
|---|
|
Direct Parent |
Hydroxybenzoic acid derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Hydroxybenzoic acid
- Benzoic acid
- Benzoyl
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Phenoxide
- Vinylogous acid
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Hydrocarbon derivative
- Organic oxygen compound
- Organic oxide
- Organooxygen compound
- Organic anion
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework |
Aromatic homomonocyclic compounds |
|---|
| External Descriptors |
- dihydroxybenzoate (CHEBI:58044)
- a monocarboxylate, a hydroxy carboxylate (CPD-633)
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
199.5 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 199.5 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 5.0 mg/mL at 5 °C | Not Available | | LogP | 1.74 | SANGSTER (1994) |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) | splash10-0a4i-0849000000-d6f7b541cabdb5e20dd6 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (3 TMS) | splash10-0a4i-3955000000-215d684d2fae395f2e76 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Negative (Annotated) | splash10-0zfr-0900000000-816a84a65cefa1b31731 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Negative (Annotated) | splash10-0a4i-1900000000-977e85749e8fa31315c5 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Negative (Annotated) | splash10-0a4i-1900000000-086d66fbe2d41a2d725c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-0udi-0900000000-e3a62163a69461ab2cff | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-0a4i-0900000000-06431a9cf0c877732fe5 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-0a4i-0900000000-e938fddf26a9bb2cd9e3 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative | splash10-0a4i-0900000000-38ef6d9660431bec9b3b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Negative | splash10-0a4i-0900000000-ac95d834c95477f325a7 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - MALDI-TOF (Voyager DE-PRO, Applied Biosystems) , Positive | splash10-004r-1913000000-eba82100caa3c2664508 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - MALDI-TOF (Voyager DE-PRO, Applied Biosystems) , Negative | splash10-0udi-0902000000-6cc114aa94b58c53c123 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - MALDI-TOF (Voyager DE-PRO, Applied Biosystems) , Positive | splash10-0k9i-0930000000-4388abc8fe86bc940e89 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - MALDI-TOF (Voyager DE-PRO, Applied Biosystems) , Negative | splash10-0udi-0901000000-077381dba9d80706a097 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - MALDI-TOF (Voyager DE-PRO, Applied Biosystems) , Positive | splash10-0fri-0930000000-4e24b1261f33fba704e2 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive | splash10-000b-9800000000-35424c6ac43370b30b89 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative | splash10-0a4i-0900000000-56fe39ec90c2e54662db | View in MoNA |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-0f79-7900000000-4ac54ba9a72c8707055d | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Ohsako M, Matsumoto Y, Goto S: Transport of aspirin and its metabolites through human erythrocyte membrane. Biol Pharm Bull. 1993 Feb;16(2):154-7. [8364451 ]
- Almaas R, Rootwelt T, Oyasaeter S, Saugstad OD: Ascorbic acid enhances hydroxyl radical formation in iron-fortified infant cereals and infant formulas. Eur J Pediatr. 1997 Jun;156(6):488-92. [9208249 ]
- Verhaeghe BJ, Lefevere MF, De Leenheer AP: Solid-phase extraction with strong anion-exchange columns for selective isolation and concentration of urinary organic acids. Clin Chem. 1988 Jun;34(6):1077-83. [3378323 ]
- Palumbo G, Carlucci G, Mazzeo P, Frieri G, Pimpo MT, Fanini D: Simultaneous determination of 5-aminosalicylic acid, acetyl-5-aminosalicylic acid and 2,5-dihydroxybenzoic acid in endoscopic intestinal biopsy samples in humans by high-performance liquid chromatography with electrochemical detection. J Pharm Biomed Anal. 1995 Dec;14(1-2):175-80. [8833980 ]
- Grootveld M, Halliwell B: Aromatic hydroxylation as a potential measure of hydroxyl-radical formation in vivo. Identification of hydroxylated derivatives of salicylate in human body fluids. Biochem J. 1986 Jul 15;237(2):499-504. [3026319 ]
- Peleg H, Noble AC: Perceptual properties of benzoic acid derivatives. Chem Senses. 1995 Aug;20(4):393-400. [8590024 ]
- Buskin JN, Upton RA, Williams RL: Improved liquid-chromatography of aspirin, salicylate, and salicyluric acid in plasma, with a modification for determining aspirin metabolites in urine. Clin Chem. 1982 May;28(5):1200-3. [7074905 ]
- Liu JH, Smith PC: Direct analysis of salicylic acid, salicyl acyl glucuronide, salicyluric acid and gentisic acid in human plasma and urine by high-performance liquid chromatography. J Chromatogr B Biomed Appl. 1996 Jan 12;675(1):61-70. [8634769 ]
- Coudray C, Talla M, Martin S, Fatome M, Favier A: High-performance liquid chromatography-electrochemical determination of salicylate hydroxylation products as an in vivo marker of oxidative stress. Anal Biochem. 1995 May 1;227(1):101-11. [7668368 ]
- Bochner F, Graham GG, Cham BE, Imhoff DM, Haavisto TM: Salicylate metabolite kinetics after several salicylates. Clin Pharmacol Ther. 1981 Aug;30(2):266-75. [7249509 ]
- Cham BE, Bochner F, Imhoff DM, Johns D, Rowland M: Simultaneous liquid-chromatographic quantitation of salicylic acid, salicyluric acid, and gentisic acid in urine. Clin Chem. 1980 Jan;26(1):111-4. [7356541 ]
|
|---|
| Synthesis Reference: |
Morris, Steward G. Preparation of gentisic acid and its fatty alcohol esters.Journal of the American Chemical Society (1949), 71 2056-7 |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|