|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110732 |
|---|
|
Identification |
|---|
| Name: |
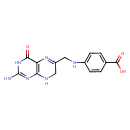
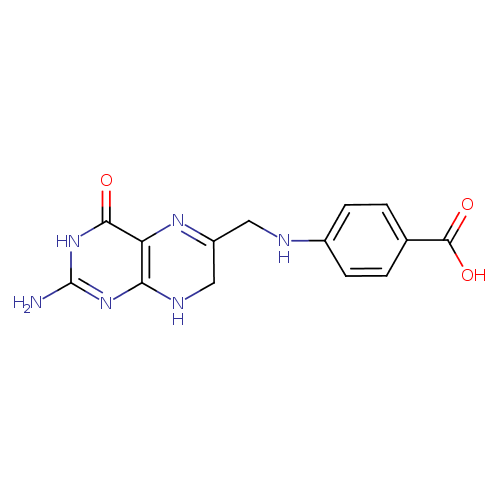
7,8-dihydropteroate |
|---|
| Description: | A pteroate that is the conjugate base of 7,8-dihydropteroic acid, arising from deprotonation of the carboxy group. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
dihydropterate
-
H2Pte
-
dihydropteroate
|
|---|
|
Chemical Formula: |
C14H13N6O3
|
|---|
| Average Molecular Weight: |
313.3 |
|---|
| Monoisotopic Molecular
Weight: |
314.1127383469 |
|---|
| InChI Key: |
WBFYVDCHGVNRBH-UHFFFAOYSA-M |
|---|
| InChI: |
InChI=1S/C14H14N6O3/c15-14-19-11-10(12(21)20-14)18-9(6-17-11)5-16-8-3-1-7(2-4-8)13(22)23/h1-4,16H,5-6H2,(H,22,23)(H4,15,17,19,20,21)/p-1 |
|---|
| CAS
number: |
2134-76-1 |
|---|
| IUPAC Name: | 4-{[(2-amino-4-oxo-3,4,7,8-tetrahydropteridin-6-yl)methyl]amino}benzoate |
|---|
|
Traditional IUPAC Name: |
7,8-dihydropteroic acid |
|---|
| SMILES: | C(NC1(=CC=C(C(=O)[O-])C=C1))C3(CNC2(=C(C(=O)NC(N)=N2)N=3)) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as pterins and derivatives. These are polycyclic aromatic compounds containing a pterin moiety, which consist of a pteridine ring bearing a ketone and an amine group to form 2-aminopteridin-4(3H)-one. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organoheterocyclic compounds |
|---|
| Sub Class | Pteridines and derivatives |
|---|
|
Direct Parent |
Pterins and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Pterin
- Aminobenzoic acid
- Aminobenzoic acid or derivatives
- Benzoic acid or derivatives
- Benzoic acid
- Benzoyl
- Aniline or substituted anilines
- Phenylalkylamine
- Hydroxypyrimidine
- Secondary aliphatic/aromatic amine
- Monocyclic benzene moiety
- Pyrimidine
- Benzenoid
- Heteroaromatic compound
- Amino acid or derivatives
- Amino acid
- Ketimine
- Azacycle
- Secondary amine
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Carboxylic acid
- Organic nitrogen compound
- Imine
- Organonitrogen compound
- Organic oxide
- Organopnictogen compound
- Amine
- Organic oxygen compound
- Organooxygen compound
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Rébeillé F, Macherel D, Mouillon JM, Garin J, Douce R (1997)Folate biosynthesis in higher plants: purification and molecular cloning of a bifunctional 6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase/7,8-dihydropteroate synthase localized in mitochondria. The EMBO journal 16, Pubmed: 9118956
|
|---|
| Synthesis Reference: |
Bartels, Rainer; Bock, Lothar. Determination of pteroic acid by high-performance thin-layer chromatography. Contribution to the investigation of 7,8-dihydropteroate synthase. Journal of Chromatography (1994), 659(1), 185-9. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|