|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110724 |
|---|
|
Identification |
|---|
| Name: |
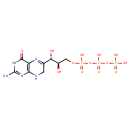
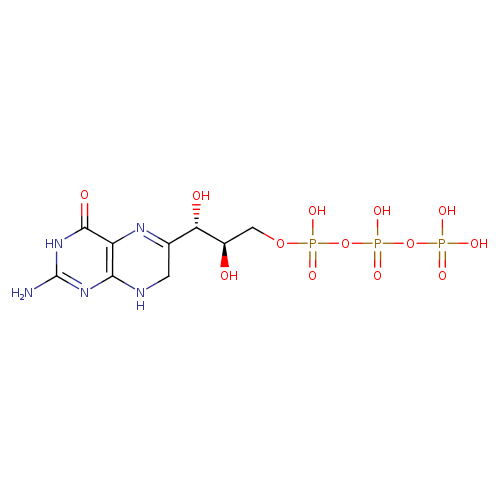
7,8-dihydroneopterin 3'-triphosphate |
|---|
| Description: | Tetraanion of 7,8-dihydroneopterin 3'-triphosphate. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
6-(L-erythro-1,2-dihydroxypropyl 3-triphosphate)-7,8-dihydropterin
-
6-[(1S,2R)-1,2-dihydroxy-3-triphosphooxypropyl]-7,8-dihydropterin
-
6-(D-erythro-1',2',3'-trihydroxypropyl)-7,8-dihydropterin-3'-triphosphate
-
7,8-dihydroneopterin 3'-triphosphate
-
2-amino-4-hydroxy-6-(erythro-1,2,3-trihydroxypropyl)dihydropteridine triphosphate
-
dihydroneopterin triphosphate
-
H2NTP
-
7,8-dihydroneopterin triphosphate
|
|---|
|
Chemical Formula: |
C9H12N5O13P3
|
|---|
| Average Molecular Weight: |
491.14 |
|---|
| Monoisotopic Molecular
Weight: |
494.9957451569 |
|---|
| InChI Key: |
DGGUVLXVLHAAGT-XINAWCOVSA-J |
|---|
| InChI: |
InChI=1S/C9H16N5O13P3/c10-9-13-7-5(8(17)14-9)12-3(1-11-7)6(16)4(15)2-25-29(21,22)27-30(23,24)26-28(18,19)20/h4,6,15-16H,1-2H2,(H,21,22)(H,23,24)(H2,18,19,20)(H4,10,11,13,14,17)/p-4/t4-,6+/m1/s1 |
|---|
| CAS
number: |
20574-65-6 |
|---|
| IUPAC Name: | (2R,3S)- 3- 3- (2- (2- amino- amino- 4- 4- oxo- oxo- 3,4,7,8- 3,4,7,8- tetrahydropteridin- tetrahydropteridin- 6- 6- yl)- yl)- 2,3- 2,3- dihydroxypropyl triphosphate dihydroxypropyl triphosphate |
|---|
|
Traditional IUPAC Name: |
{[(2R,3S)-3-(2-amino-4-oxo-7,8-dihydro-3H-pteridin-6-yl)-2,3-dihydroxypropoxy(hydroxy)phosphoryl]oxy(hydroxy)phosphoryl}oxyphosphonic acid |
|---|
| SMILES: | C1(NC2(N=C(N)NC(=O)C(N=C1C(O)C(O)COP([O-])(=O)OP([O-])(=O)OP([O-])(=O)[O-])=2)) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as biopterins and derivatives. These are coenzymes containing a 2-amino-pteridine-4-one derivative. They are mainly synthesized in several parts of the body, including the pineal gland. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organoheterocyclic compounds |
|---|
| Sub Class | Pteridines and derivatives |
|---|
|
Direct Parent |
Biopterins and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Biopterin
- Secondary aliphatic/aromatic amine
- Monoalkyl phosphate
- Hydroxypyrimidine
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Pyrimidine
- Alkyl phosphate
- Heteroaromatic compound
- Ketimine
- Secondary alcohol
- 1,2-diol
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Azacycle
- Organic oxide
- Alcohol
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Imine
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -4 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Curtius HC, Heintel D, Ghisla S, Kuster T, Leimbacher W, Niederwieser A: Biosynthesis of tetrahydrobiopterin in man. J Inherit Metab Dis. 1985;8 Suppl 1:28-33. [3930838 ]
|
|---|
| Synthesis Reference: |
Ferre, Juan; Naylor, Edwin W.; Jacobson, K. Bruce. Repetitive recycling of guanosine triphosphate cyclohydrolase I for synthesis of dihydroneopterin triphosphate. Analytical Biochemistry (1989), 176(1), 15-18. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|