|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110716 |
|---|
|
Identification |
|---|
| Name: |
precorrin-3B |
|---|
| Description: | Heptacarboxylate anion of precorrin-3B. |
|---|
|
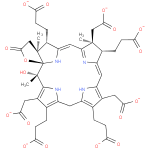
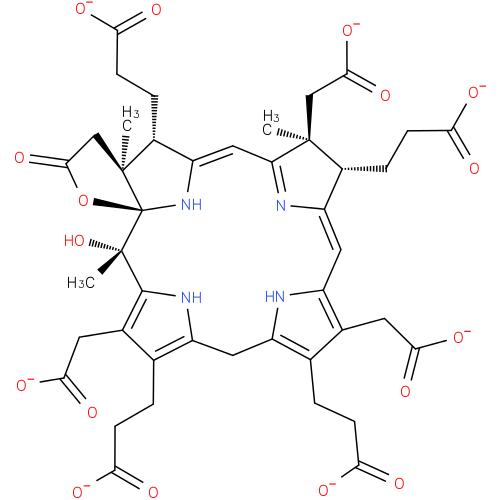
Structure |
|
|---|
| Synonyms: | |
|---|
|
Chemical Formula: |
C43H43N4O17
|
|---|
| Average Molecular Weight: |
887.83 |
|---|
| Monoisotopic Molecular
Weight: |
894.3170962015 |
|---|
| InChI Key: |
KJHZYYJBHKAUHS-NXWQJPGNSA-G |
|---|
| InChI: |
InChI=1S/C43H50N4O17/c1-40(17-37(60)61)23(6-10-33(52)53)28-15-27-21(12-35(56)57)19(4-8-31(48)49)25(44-27)14-26-20(5-9-32(50)51)22(13-36(58)59)39(46-26)42(3,63)43-41(2,18-38(62)64-43)24(7-11-34(54)55)29(47-43)16-30(40)45-28/h15-16,23-24,44,46-47,63H,4-14,17-18H2,1-3H3,(H,48,49)(H,50,51)(H,52,53)(H,54,55)(H,56,57)(H,58,59)(H,60,61)/p-7/b28-15-,29-16-/t23-,24-,40+,41+,42+,43-/m1/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 3,3',3'',3'''- (7S,8S,12S,13S,14R,15S)- (7S,8S,12S,13S,14R,15S)- 2,7,12,18- 2,7,12,18- tetrakis(2- tetrakis(2- carboxylatoethyl)- carboxylatoethyl)- 3,8,17- 3,8,17- tris(carboxylatomethyl)- tris(carboxylatomethyl)- 15- 15- hydroxy- hydroxy- 8,13,15- 8,13,15- trimethyl- trimethyl- 7,8,12,13,14,15,20,24- 7,8,12,13,14,15,20,24- octahydroporphyrin- octahydroporphyrin- 131,14- 131,14- carbolactone carbolactone |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | CC4(CC(=O)[O-])(C(C3(=CC1(=C(CC(=O)[O-])C(CCC(=O)[O-])=C(N1)CC2(=C(CCC(=O)[O-])C(CC(=O)[O-])=C(N2)C(O)(C)C56(OC(CC(C)(C(C(=CC(=N3)4)N5)CCC(=O)[O-])6)=O)))))CCC(=O)[O-]) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as tetrapyrroles and derivatives. These are polycyclic aromatic compounds containing four pyrrole rings joined by one-carbon units linking position 2 of one pyrrole ring to position 5 of the next. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
|
Class |
Tetrapyrroles and derivatives |
|---|
| Sub Class | Not Available |
|---|
|
Direct Parent |
Tetrapyrroles and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Tetrapyrrole skeleton
- Gamma butyrolactone
- Substituted pyrrole
- Heteroaromatic compound
- Pyrrole
- Pyrrolidine
- Pyrroline
- Tetrahydrofuran
- Tertiary alcohol
- Amino acid or derivatives
- Carboxylic acid ester
- Ketimine
- Amino acid
- Lactone
- Carboxylic acid derivative
- Carboxylic acid
- Secondary aliphatic amine
- Enamine
- Oxacycle
- Azacycle
- Secondary amine
- Organic oxide
- Imine
- Organopnictogen compound
- Organic nitrogen compound
- Amine
- Hydrocarbon derivative
- Organonitrogen compound
- Organooxygen compound
- Carbonyl group
- Alcohol
- Organic oxygen compound
- Organic anion
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -7 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- adenosylcobalamin biosynthesis II (late cobalt incorporation)P381-PWY
- cob(II)yrinate a,c-diamide biosynthesis II (late cobalt incorporation)PWY-7376
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|