|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110710 |
|---|
|
Identification |
|---|
| Name: |
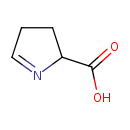
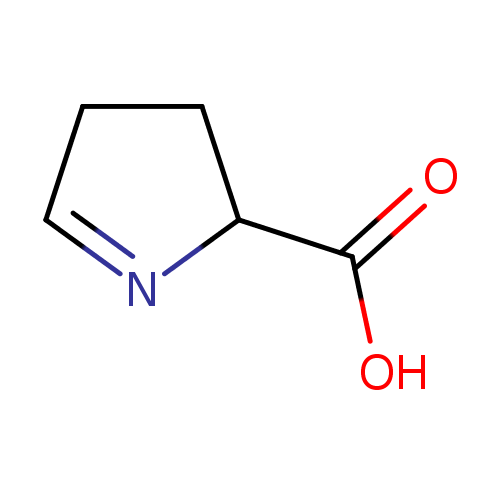
(S)-1-pyrroline-5-carboxylate |
|---|
| Description: | A 1-pyrroline-5-carboxylate resulting from the removal of the proton from the carboxy group of (S)-1-pyrroline-5-carboxylic acid. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
pyrroline 5-carboxylate
-
L-Δ1-pyrroline-5-carboxylate
-
1-pyrroline-5-carboxylate
-
L-1-pyrroline-5-carboxylate
-
δ-1-pyrroline-5-carboxylic acid
-
δ-1-pyrroline-5-carboxylate
|
|---|
|
Chemical Formula: |
C5H6NO2
|
|---|
| Average Molecular Weight: |
112.11 |
|---|
| Monoisotopic Molecular
Weight: |
113.0476784741 |
|---|
| InChI Key: |
DWAKNKKXGALPNW-BYPYZUCNSA-M |
|---|
| InChI: |
InChI=1S/C5H7NO2/c7-5(8)4-2-1-3-6-4/h3-4H,1-2H2,(H,7,8)/p-1/t4-/m0/s1 |
|---|
| CAS
number: |
2906-39-0 |
|---|
| IUPAC Name: | (2S)-3,4-dihydro-2H-pyrrole-2-carboxylate |
|---|
|
Traditional IUPAC Name: |
1-pyrroline-5-carboxylic acid |
|---|
| SMILES: | C1(=NC(CC1)C(=O)[O-]) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as alpha amino acids and derivatives. These are amino acids in which the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (alpha carbon), or a derivative thereof. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Carboxylic acids and derivatives |
|---|
|
Direct Parent |
Alpha amino acids and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Alpha-amino acid or derivatives
- Pyrroline carboxylic acid
- Pyrroline carboxylic acid or derivatives
- Pyrroline
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Propargyl-type 1,3-dipolar organic compound
- Organic 1,3-dipolar compound
- Organoheterocyclic compound
- Azacycle
- Organopnictogen compound
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Carbonyl group
- Imine
- Organic nitrogen compound
- Hydrocarbon derivative
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Simila S: Hydroxyproline metabolism in type II hyperprolinaemia. Ann Clin Biochem. 1979 Jul;16(4):177-81. [533224 ]
- Humbertclaude V, Rivier F, Roubertie A, Echenne B, Bellet H, Vallat C, Morin D: Is hyperprolinemia type I actually a benign trait? Report of a case with severe neurologic involvement and vigabatrin intolerance. J Child Neurol. 2001 Aug;16(8):622-3. [11510941 ]
- Mixson AJ, Phang JM: The uptake of pyrroline 5-carboxylate. Group translocation mediating the transfer of reducing-oxidizing potential. J Biol Chem. 1988 Aug 5;263(22):10720-4. [3392037 ]
- Wakabayashi Y: Tissue-selective expression of enzymes of arginine synthesis. Curr Opin Clin Nutr Metab Care. 1998 Jul;1(4):335-9. [10565370 ]
- Onenli-Mungan N, Yuksel B, Elkay M, Topaloglu AK, Baykal T, Ozer G: Type II hyperprolinemia: a case report. Turk J Pediatr. 2004 Apr-Jun;46(2):167-9. [15214748 ]
- Fleming GA, Hagedorn CH, Granger AS, Phang JM: Pyrroline-5-carboxylate in human plasma. Metabolism. 1984 Aug;33(8):739-42. [6748947 ]
|
|---|
| Synthesis Reference: |
Vogel, Henry J.; Davis, Bernard D. Glutamic g-semialdehyde and D1-pyrroline-5-carboxylic acid, intermediates in the biosynthesis of proline. Journal of the American Chemical Society (1952), 74 109-12. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|