|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110705 |
|---|
|
Identification |
|---|
| Name: |
FAD |
|---|
| Description: | A flavin adenine dinucleotide in which the substituent at position 10 of the flavin nucleus is a 5'-adenosyldiphosphoribityl group. |
|---|
|
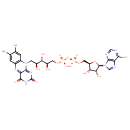
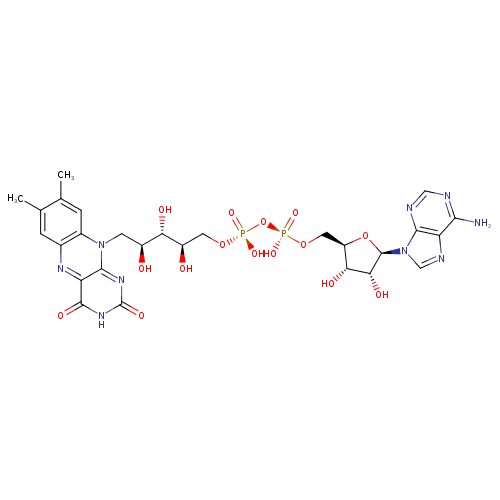
Structure |
|
|---|
| Synonyms: | -
flavin adenine dinucleotide oxidized
-
flavin adenine dinucleotide
-
flavitan
|
|---|
|
Chemical Formula: |
C27H30N9O15P2
|
|---|
| Average Molecular Weight: |
782.53 |
|---|
| Monoisotopic Molecular
Weight: |
785.1571344576 |
|---|
| InChI Key: |
IMGVNJNCCGXBHD-UYBVJOGSSA-K |
|---|
| InChI: |
InChI=1S/C27H33N9O15P2/c1-10-3-12-13(4-11(10)2)35(24-18(32-12)25(42)34-27(43)33-24)5-14(37)19(39)15(38)6-48-52(44,45)51-53(46,47)49-7-16-20(40)21(41)26(50-16)36-9-31-17-22(28)29-8-30-23(17)36/h3-4,8-9,14-16,19-21,26,37-41H,5-7H2,1-2H3,(H5,28,29,30,34,42,43,44,45,46,47)/p-3/t14-,15+,16+,19-,20+,21+,26+/m0/s1 |
|---|
| CAS
number: |
146-14-5 |
|---|
| IUPAC Name: | adenosine 5'- (3- (3- {D- {D- ribo- ribo- 5- 5- [7,8- [7,8- dimethyl- dimethyl- 2,4- 2,4- dioxo- dioxo- 3,4- 3,4- dihydrobenzo[g]pteridin- dihydrobenzo[g]pteridin- 10(2H)- 10(2H)- yl]- yl]- 2,3,4- 2,3,4- trihydroxypentyl} dihydrogen diphosphate) trihydroxypentyl} dihydrogen diphosphate) |
|---|
|
Traditional IUPAC Name: |
flavine-adenine dinucleotide |
|---|
| SMILES: | CC6(=C(C)C=C5(C(N=C1(C(=O)[N-]C(=O)N=C1N(CC(C(O)C(O)COP(OP([O-])(OCC4(C(O)C(O)C(N3(C=NC2(C(N)=NC=NC=23)))O4))=O)([O-])=O)O)5))=C6)) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as flavin nucleotides. These are nucleotides containing a flavin moiety. Flavin is a compound that contains the tricyclic isoalloxazine ring system, which bears 2 oxo groups at the 2- and 4-positions. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Nucleosides, nucleotides, and analogues |
|---|
| Sub Class | Flavin nucleotides |
|---|
|
Direct Parent |
Flavin nucleotides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Flavin nucleotide
- (3'->5')-dinucleotide
- (3'->5')-dinucleotide or analogue
- Purine ribonucleoside diphosphate
- Purine ribonucleoside monophosphate
- Flavin
- Pentose phosphate
- Pentose-5-phosphate
- Isoalloxazine
- Glycosyl compound
- N-glycosyl compound
- Organic pyrophosphate
- Diazanaphthalene
- 6-aminopurine
- Pentose monosaccharide
- Monosaccharide phosphate
- Pteridine
- Quinoxaline
- Imidazopyrimidine
- Purine
- Pyrimidone
- Monoalkyl phosphate
- Aminopyrimidine
- N-substituted imidazole
- Pyrimidine
- Benzenoid
- Pyrazine
- Alkyl phosphate
- Organic phosphoric acid derivative
- Primary aromatic amine
- Imidolactam
- Phosphoric acid ester
- Monosaccharide
- Heteroaromatic compound
- Imidazole
- Oxolane
- Vinylogous amide
- Azole
- Secondary alcohol
- Lactam
- Polyol
- Organoheterocyclic compound
- Azacycle
- Oxacycle
- Alcohol
- Primary amine
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Amine
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 5 mg/mL | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-000i-0001200900-b0740b3d33d50996ccde | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-000m-0105900000-3abff26d5bb35f8527b8 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-000i-0931700000-73f360589a13230eea7a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-000j-0908600300-b1eccda8ebae8b9e4cc1 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-000i-0900000000-bdb826f9c3cbc09eff9b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-00di-0019800000-00ad56b6b6a3bb4516e6 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-000b-0009400000-667064ca470a5c341974 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-000j-0509700500-8766cb874f927ed5a795 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-000i-0900000000-3125e04c09a14c62f22a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-00di-0019700000-78c46ea4b4f562757e56 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-000b-0009400000-94b845d2c8d082b9484c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-001i-0000100900-6bac7b7f631dfc074a0c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-001i-0920000000-49c9d4fb9b57a59f45a5 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-000i-0003900000-572f0b1bd59f71ab3c5f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-000i-0003900000-d2ede6a2e7183f1c32a3 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-001i-0000100900-78554afbc26abe26af35 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-001i-0930000000-274da0a01c651b791733 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-000i-0003900000-55939cda9e14fc582757 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-000i-0003900000-db784762434fac3d351f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,1H] 2D NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Ravi G, Venkatesh YP (2014)Recognition of riboflavin and the capsular polysaccharide of Haemophilus influenzae type b by antibodies generated to the haptenic epitope D-ribitol. Glycoconjugate journal 31, Pubmed: 24643482
- Ravi G, Venkatesh YP (2014)Recognition of flavin mononucleotide, Haemophilus influenzae type b and its capsular polysaccharide vaccines by antibodies specific to D-ribitol-5-phosphate. Glycoconjugate journal 31, Pubmed: 25108762
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|