|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110692 |
|---|
|
Identification |
|---|
| Name: |
octanal |
|---|
| Description: | A saturated fatty aldehyde formally arising from reduction of the carboxy group of caprylic acid (octanoic acid). |
|---|
|
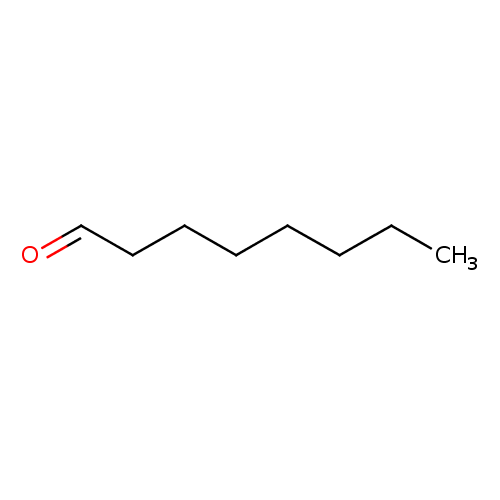
Structure |
|
|---|
| Synonyms: | -
1-Octaldehyde
-
1-Octylaldehyde
-
1-Caprylaldehyde
-
1-caprylaldehyde
-
1-octylaldehyde
-
1-octaldehyde
-
1-octanal
-
n-octanal
|
|---|
|
Chemical Formula: |
C8H16O
|
|---|
| Average Molecular Weight: |
128.21 |
|---|
| Monoisotopic Molecular
Weight: |
128.1201151357 |
|---|
| InChI Key: |
NUJGJRNETVAIRJ-UHFFFAOYSA-N |
|---|
| InChI: |
InChI=1S/C8H16O/c1-2-3-4-5-6-7-8-9/h8H,2-7H2,1H3 |
|---|
| CAS
number: |
124-13-0 |
|---|
| IUPAC Name: | octanal |
|---|
|
Traditional IUPAC Name: |
octanal |
|---|
| SMILES: | CCCCCCC[CH]=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as medium-chain aldehydes. These are an aldehyde with a chain length containing between 6 and 12 carbon atoms. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic oxygen compounds |
|---|
| Sub Class | Organooxygen compounds |
|---|
|
Direct Parent |
Medium-chain aldehydes |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Medium-chain aldehyde
- Alpha-hydrogen aldehyde
- Organic oxide
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Liquid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
-23 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | -23 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 0.56 mg/mL | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS | Not Available |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI RMU-6M) , Positive | splash10-052f-9000000000-afbedb8b18342773ec25 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI M-80B) , Positive | splash10-052f-9000000000-5116ccc02f51ccb0e86a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - EI-B (HP 5970) , Positive | splash10-052f-9000000000-e20fdcbb6710ee07f303 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI RMU-6M) , Positive | splash10-000x-9000000000-70ec440a61fb95915131 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-1900000000-e4de633fb7345cb25ac3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01t9-9600000000-ffa4810bd6f0997adbe2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-9000000000-0663d075e83465e0b94a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0900000000-54dee15774147b509eee | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-3900000000-0c41e22b27b06ecc562d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-7cb58e4c39970a374f81 | View in MoNA |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-052f-9000000000-23d54ae7b45c372a7325 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Song MK, Lee HS, Choi HS, Shin CY, Kim YJ, Park YK, Ryu JC (2014)Octanal-induced inflammatory responses in cells relevant for lung toxicity: expression and release of cytokines in A549 human alveolar cells. Human & experimental toxicology 33, Pubmed: 24130214
- Sansone-Land A, Takeoka GR, Shoemaker CF (2014)Volatile constituents of commercial imported and domestic black-ripe table olives (Olea europaea). Food chemistry 149, Pubmed: 24295708
- Mathure SV, Jawali N, Thengane RJ, Nadaf AB (2014)Comparative quantitative analysis of headspace volatiles and their association with BADH2 marker in non-basmati scented, basmati and non-scented rice (Oryza sativa L.) cultivars of India. Food chemistry 142, Pubmed: 24001856
|
|---|
| Synthesis Reference: |
Yokoyama, Toshiharu; Matsuyama, Naoko; Maki, Takao. Preparation of aldehydes. Jpn. Kokai Tokkyo Koho (1992), 6 pp. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|