|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110690 |
|---|
|
Identification |
|---|
| Name: |
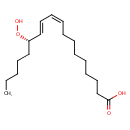
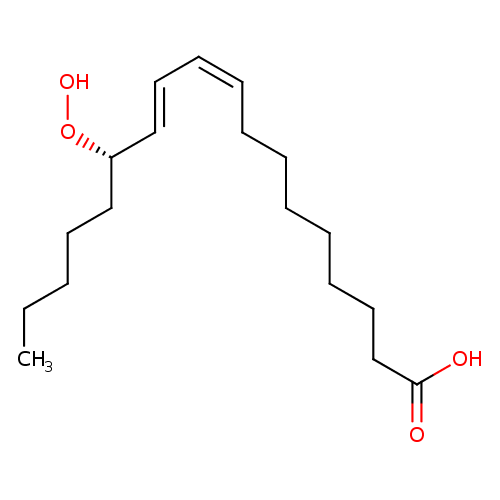
(13S)-HPODE |
|---|
| Description: | The (S)-enantiomer of 13-HPODE |
|---|
|
Structure |
|
|---|
| Synonyms: | -
13(S)-hydroperoxy-9(Z),11(E)-octadecadienoate
-
(9Z,11E)-(13S)-13-hydroperoxyoctadeca-9,11-dienoate
|
|---|
|
Chemical Formula: |
C18H31O4
|
|---|
| Average Molecular Weight: |
311.44 |
|---|
| Monoisotopic Molecular
Weight: |
312.2300595156 |
|---|
| InChI Key: |
JDSRHVWSAMTSSN-IRQZEAMPSA-M |
|---|
| InChI: |
InChI=1S/C18H32O4/c1-2-3-11-14-17(22-21)15-12-9-7-5-4-6-8-10-13-16-18(19)20/h7,9,12,15,17,21H,2-6,8,10-11,13-14,16H2,1H3,(H,19,20)/p-1/b9-7-,15-12+/t17-/m0/s1 |
|---|
| CAS
number: |
33964-75-9 |
|---|
| IUPAC Name: | (9Z,11E,13S)-13-hydroperoxyoctadeca-9,11-dienoic acid |
|---|
|
Traditional IUPAC Name: |
13-HpODE |
|---|
| SMILES: | CCCCCC(C=CC=CCCCCCCCC(=O)[O-])OO |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as lineolic acids and derivatives. These are derivatives of lineolic acid. Lineolic acid is a polyunsaturated omega-6 18 carbon long fatty acid, with two CC double bonds at the 9- and 12-positions. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Lipids and lipid-like molecules |
|---|
| Sub Class | Fatty Acyls |
|---|
|
Direct Parent |
Lineolic acids and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Octadecanoid
- Long-chain fatty acid
- Hydroperoxy fatty acid
- Fatty acid
- Unsaturated fatty acid
- Allylic hydroperoxide
- Hydroperoxide
- Carboxylic acid derivative
- Alkyl hydroperoxide
- Carboxylic acid
- Peroxol
- Monocarboxylic acid or derivatives
- Carbonyl group
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Kuhn H: Biosynthesis, metabolization and biological importance of the primary 15-lipoxygenase metabolites 15-hydro(pero)XY-5Z,8Z,11Z,13E-eicosatetraenoic acid and 13-hydro(pero)XY-9Z,11E-octadecadienoic acid. Prog Lipid Res. 1996 Sep;35(3):203-26. [9082450 ]
|
|---|
| Synthesis Reference: |
Baba, N.; Yoneda, K.; Iwasa, J.; Tahara, S. Asymmetric synthesis of diacylglycerophosphocholine hydroperoxide VIa, lipoxygenase-catalyzed hydroperoxidation of linoleic acid and lipase-catalyzed enantioselective stearoylation of 2-O-benzoyl-1,3-propanediol. Indian Journal of Chemistry, Section B: Organic Chemistry Including Medicinal Chemistry (1992), 31B(12), 824-7. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|