|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110677 |
|---|
|
Identification |
|---|
| Name: |
choline sulfate |
|---|
| Description: | An ammonium betaine that is the conjugate base of choline hydrogen sulfate, obtained by deprotonation of the sulfate OH group. |
|---|
|
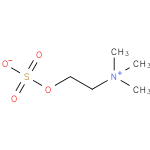
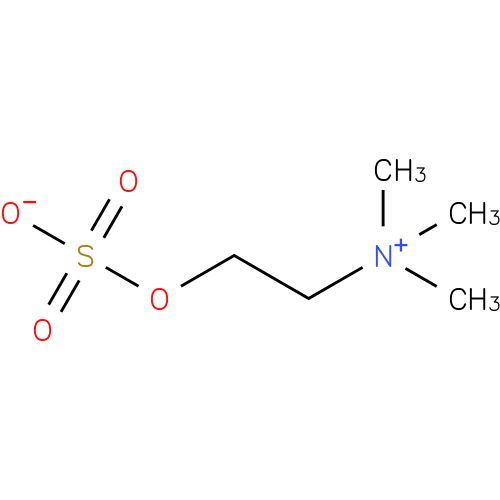
Structure |
|
|---|
| Synonyms: | |
|---|
|
Chemical Formula: |
C5H13NO4S
|
|---|
| Average Molecular Weight: |
183.22 |
|---|
| Monoisotopic Molecular
Weight: |
184.064353633 |
|---|
| InChI Key: |
WXCQAWGXWVRCGP-UHFFFAOYSA-N |
|---|
| InChI: |
InChI=1S/C5H13NO4S/c1-6(2,3)4-5-10-11(7,8)9/h4-5H2,1-3H3 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 2-(trimethylammonio)ethyl sulfate |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | C[N+](CCOS(=O)(=O)[O-])(C)C |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as sulfuric acid monoesters. These are organic compounds containing the sulfuric acid monoester functional group, with the generic structure ROS(O)(=O)=O, (R=organyl group). |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
|
Class |
Organic sulfuric acids and derivatives |
|---|
| Sub Class | Sulfuric acid esters |
|---|
|
Direct Parent |
Sulfuric acid monoesters |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Alkyl sulfate
- Sulfate-ester
- Sulfuric acid monoester
- Tetraalkylammonium salt
- Quaternary ammonium salt
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organic salt
- Organooxygen compound
- Organonitrogen compound
- Amine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
- ammonium betaine, choline sulfates (CHEBI:16822)
- a small molecule (CPD-543)
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Fitzgerald JW, Luschinski PC (1977)Further studies on the formation of choline sulfate by bacteria. Canadian journal of microbiology 23, Pubmed: 871964
- KAJI A, McELROY WD (1958)Enzymic formation of choline sulfate. Biochimica et biophysica acta 30, Pubmed: 13584416
- Bakkerud KG, Nissen P (1980)Bacteria-mediated uptake of choline sulfate by plants: bacterial effectiveness. Biochimica et biophysica acta 600, Pubmed: 7397169
- Nissen P, Benson AA (1961)Choline Sulfate in Higher Plants. Science (New York, N.Y.) 134, Pubmed: 17779079
- ITAHASHI M (1961)Comparative biochemistry of choline sulfate metabolism. Journal of biochemistry 50, Pubmed: 13718099
- Nissen P, Benson AA (1964)Active Transport of Choline Sulfate by Barley Roots. Plant physiology 39, Pubmed: 16655967
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|