|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110634 |
|---|
|
Identification |
|---|
| Name: |
3-hydroxy-L-kynurenine |
|---|
| Description: | Zwitterionic form of 3-hydroxy-L-kynurenine arising from transfer of a proton from the carboxy to the amino group; major species at pH 7.3 |
|---|
|
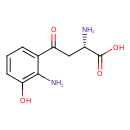
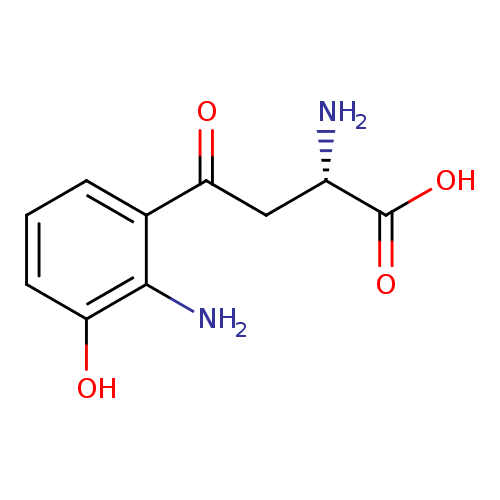
Structure |
|
|---|
| Synonyms: | -
L-3-hydroxykynurenine
-
3-hydroxy-kynurenine
|
|---|
|
Chemical Formula: |
C10H12N2O4
|
|---|
| Average Molecular Weight: |
224.22 |
|---|
| Monoisotopic Molecular
Weight: |
225.0875319161 |
|---|
| InChI Key: |
VCKPUUFAIGNJHC-LURJTMIESA-N |
|---|
| InChI: |
InChI=1S/C10H12N2O4/c11-6(10(15)16)4-8(14)5-2-1-3-7(13)9(5)12/h1-3,6,13H,4,11-12H2,(H,15,16)/t6-/m0/s1 |
|---|
| CAS
number: |
606-14-4 |
|---|
| IUPAC Name: | (2S)-4-(2-amino-3-hydroxyphenyl)-2-azaniumyl-4-oxobutanoate |
|---|
|
Traditional IUPAC Name: |
3-hydroxy-L-kynurenine |
|---|
| SMILES: | C([O-])(=O)C([N+])CC(=O)C1(=C(N)C(O)=CC=C1) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as alkyl-phenylketones. These are aromatic compounds containing a ketone substituted by one alkyl group, and a phenyl group. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic oxygen compounds |
|---|
| Sub Class | Organooxygen compounds |
|---|
|
Direct Parent |
Alkyl-phenylketones |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Alkyl-phenylketone
- Butyrophenone
- Alpha-amino acid
- Alpha-amino acid or derivatives
- L-alpha-amino acid
- O-aminophenol
- Aminophenol
- Benzoyl
- Aniline or substituted anilines
- Gamma-keto acid
- Aryl alkyl ketone
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Monocyclic benzene moiety
- Beta-aminoketone
- Keto acid
- Benzenoid
- Primary aromatic amine
- Vinylogous amide
- Amino acid
- Amino acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Primary aliphatic amine
- Amine
- Hydrocarbon derivative
- Organopnictogen compound
- Organic nitrogen compound
- Organonitrogen compound
- Primary amine
- Organic oxide
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework |
Aromatic homomonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Sardar AM, Bell JE, Reynolds GP: Increased concentrations of the neurotoxin 3-hydroxykynurenine in the frontal cortex of HIV-1-positive patients. J Neurochem. 1995 Feb;64(2):932-5. [7830088 ]
- Guidetti P, Schwarcz R: 3-Hydroxykynurenine potentiates quinolinate but not NMDA toxicity in the rat striatum. Eur J Neurosci. 1999 Nov;11(11):3857-63. [10583474 ]
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|