|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110631 |
|---|
|
Identification |
|---|
| Name: |
N-acetyl-L-glutamate |
|---|
| Description: | An N-acyl-L-α-amino acid anion resulting from deprotonation of both carboxy groups of N-acetyl-L-glutamic acid. |
|---|
|
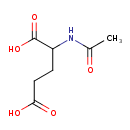
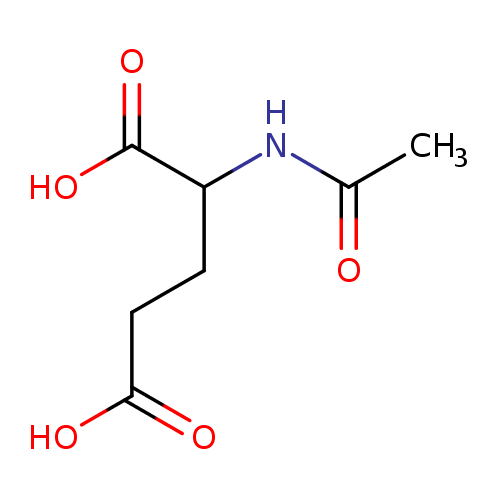
Structure |
|
|---|
| Synonyms: | -
N-acetyl-L-glutamic acid
-
acetyl-L-glu
-
acetyl-L-glutamate
-
NAG
|
|---|
|
Chemical Formula: |
C7H9NO5
|
|---|
| Average Molecular Weight: |
187.15 |
|---|
| Monoisotopic Molecular
Weight: |
189.0637224688 |
|---|
| InChI Key: |
RFMMMVDNIPUKGG-YFKPBYRVSA-L |
|---|
| InChI: |
InChI=1S/C7H11NO5/c1-4(9)8-5(7(12)13)2-3-6(10)11/h5H,2-3H2,1H3,(H,8,9)(H,10,11)(H,12,13)/p-2/t5-/m0/s1 |
|---|
| CAS
number: |
1188-37-0 |
|---|
| IUPAC Name: | (2S)-2-acetamidopentanedioate |
|---|
|
Traditional IUPAC Name: |
acetylglutamate |
|---|
| SMILES: | CC(=O)NC(C([O-])=O)CCC(=O)[O-] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as glutamic acid and derivatives. These are compounds containing glutamic acid or a derivative thereof resulting from reaction of glutamic acid at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Carboxylic acids and derivatives |
|---|
|
Direct Parent |
Glutamic acid and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Glutamic acid or derivatives
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- Dicarboxylic acid or derivatives
- Fatty acid
- Acetamide
- Secondary carboxylic acid amide
- Carboxamide group
- Carboxylic acid
- Hydrocarbon derivative
- Carbonyl group
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxide
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
199 - 201 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 199 - 201 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 52 mg/mL | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Pekkala S, Martínez AI, Barcelona B, Gallego J, Bendala E, Yefimenko I, Rubio V, Cervera J (2009)Structural insight on the control of urea synthesis: identification of the binding site for N-acetyl-L-glutamate, the essential allosteric activator of mitochondrial carbamoyl phosphate synthetase. The Biochemical journal 424, Pubmed: 19754428
- Jia X, Ozawa K, Loscha K, Otting G (2009)Glutarate and N-acetyl-L-glutamate buffers for cell-free synthesis of selectively 15N-labelled proteins. Journal of biomolecular NMR 44, Pubmed: 19399372
|
|---|
| Synthesis Reference: |
hang, Xiaolin; Yang, Qiyong; Sun, Yuesheng. Preparation of N-acetyl-L-glutamic acid. Huaxue Shijie (2002), 43(7), 363-365. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|