|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110630 |
|---|
|
Identification |
|---|
| Name: |
N-formimino-L-glutamate |
|---|
| Description: | Conjugate base of N-formimidoyl-L-glutamic acid having both carboxy groups in anionic form and the imine nitrogen protonated. |
|---|
|
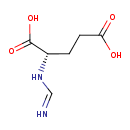
Structure |
|
|---|
| Synonyms: | -
N-formimino-glutamate
-
formiminoglutamate
-
formimino-glu
-
α-formamidinoglutarate
-
N-formimidoyl-L-glutamate
|
|---|
|
Chemical Formula: |
C6H9N2O4
|
|---|
| Average Molecular Weight: |
173.15 |
|---|
| Monoisotopic Molecular
Weight: |
175.0718818519 |
|---|
| InChI Key: |
NRXIKWMTVXPVEF-BYPYZUCNSA-M |
|---|
| InChI: |
InChI=1S/C6H10N2O4/c7-3-8-4(6(11)12)1-2-5(9)10/h3-4H,1-2H2,(H2,7,8)(H,9,10)(H,11,12)/p-1/t4-/m0/s1 |
|---|
| CAS
number: |
816-90-0 |
|---|
| IUPAC Name: | (2S)-2-[(iminiumylmethyl)amino]pentanedioate |
|---|
|
Traditional IUPAC Name: |
N-formimino-L-glutamate |
|---|
| SMILES: | [CH](=[N+])NC(C([O-])=O)CCC([O-])=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as glutamic acid and derivatives. These are compounds containing glutamic acid or a derivative thereof resulting from reaction of glutamic acid at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Carboxylic acids and derivatives |
|---|
|
Direct Parent |
Glutamic acid and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Glutamic acid or derivatives
- Dicarboxylic acid or derivatives
- Fatty acid
- Amidine
- Carboxylic acid amidine
- Carboxylic acid
- Carboximidamide
- Propargyl-type 1,3-dipolar organic compound
- Organic 1,3-dipolar compound
- Formamidine
- Organooxygen compound
- Carbonyl group
- Organic nitrogen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organic oxygen compound
- Organic oxide
- Organopnictogen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Hilton JF, Christensen KE, Watkins D, Raby BA, Renaud Y, de la Luna S, Estivill X, MacKenzie RE, Hudson TJ, Rosenblatt DS: The molecular basis of glutamate formiminotransferase deficiency. Hum Mutat. 2003 Jul;22(1):67-73. [12815595 ]
- Haurani FI, Hall CA, Rubin R: Megaloblastic anemia as a result of an abnormal transcobalamin II (Cardeza). J Clin Invest. 1979 Nov;64(5):1253-9. [500809 ]
- Perry TL, Applegarth DA, Evans ME, Hansen S, Jellum E: Metabolic studies of a family with massive formiminoglutamic aciduria. Pediatr Res. 1975 Mar;9(3):117-22. [235753 ]
- Verhoeven NM, Wanders RJ, Poll-The BT, Saudubray JM, Jakobs C: The metabolism of phytanic acid and pristanic acid in man: a review. J Inherit Metab Dis. 1998 Oct;21(7):697-728. [9819701 ]
|
|---|
| Synthesis Reference: |
Tabor, Herbert; Rabinowitz, Jesse C. Insts. Formiminoglycine, formimino-L-aspartic acid, and formimino-L-glutamic acid. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|