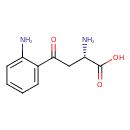
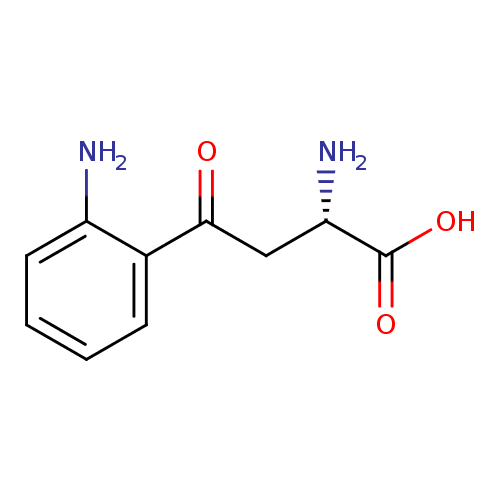
| References: |
- Medana IM, Day NP, Salahifar-Sabet H, Stocker R, Smythe G, Bwanaisa L, Njobvu A, Kayira K, Turner GD, Taylor TE, Hunt NH: Metabolites of the kynurenine pathway of tryptophan metabolism in the cerebrospinal fluid of Malawian children with malaria. J Infect Dis. 2003 Sep 15;188(6):844-9. Epub 2003 Sep 9. [12964115 ]
- Widner B, Sepp N, Kowald E, Ortner U, Wirleitner B, Fritsch P, Baier-Bitterlich G, Fuchs D: Enhanced tryptophan degradation in systemic lupus erythematosus. Immunobiology. 2000 Apr;201(5):621-30. [10834318 ]
- Widner B, Sepp N, Kowald E, Kind S, Schmuth M, Fuchs D: Degradation of tryptophan in patients with systemic lupus erythematosus. Adv Exp Med Biol. 1999;467:571-7. [10721102 ]
- Joseph MH: Determination of kynurenine by a simple gas-liquid chromatographic method applicable to urine, plasma, brain and cerebrospinal fluid. J Chromatogr. 1978 Jul 1;146(1):33-41. [670356 ]
- Heyes MP, Saito K, Lackner A, Wiley CA, Achim CL, Markey SP: Sources of the neurotoxin quinolinic acid in the brain of HIV-1-infected patients and retrovirus-infected macaques. FASEB J. 1998 Jul;12(10):881-96. [9657528 ]
- Fujigaki S, Saito K, Takemura M, Fujii H, Wada H, Noma A, Seishima M: Species differences in L-tryptophan-kynurenine pathway metabolism: quantification of anthranilic acid and its related enzymes. Arch Biochem Biophys. 1998 Oct 15;358(2):329-35. [9784247 ]
- Widner B, Werner ER, Schennach H, Fuchs D: An HPLC method to determine tryptophan and kynurenine in serum simultaneously. Adv Exp Med Biol. 1999;467:827-32. [10721136 ]
- Saito K, Fujigaki S, Heyes MP, Shibata K, Takemura M, Fujii H, Wada H, Noma A, Seishima M: Mechanism of increases in L-kynurenine and quinolinic acid in renal insufficiency. Am J Physiol Renal Physiol. 2000 Sep;279(3):F565-72. [10966936 ]
- Heyes MP, Saito K, Milstien S, Schiff SJ: Quinolinic acid in tumors, hemorrhage and bacterial infections of the central nervous system in children. J Neurol Sci. 1995 Nov;133(1-2):112-8. [8583213 ]
- Martinsons A, Rudzite V, Groma V, Bratslavska O, Widner B, Fuchs D: Kynurenine and neopterin in chronic glomerulonephritis. Adv Exp Med Biol. 1999;467:579-86. [10721103 ]
- Heyes MP, Saito K, Crowley JS, Davis LE, Demitrack MA, Der M, Dilling LA, Elia J, Kruesi MJ, Lackner A, et al.: Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain. 1992 Oct;115 ( Pt 5):1249-73. [1422788 ]
- Moroni F, Carpenedo R, Chiarugi A: Kynurenine hydroxylase and kynureninase inhibitors as tools to study the role of kynurenine metabolites in the central nervous system. Adv Exp Med Biol. 1996;398:203-10. [8906267 ]
- Calandra P: Research on tryptophan metabolites "via kynurenine" in epidermis of man and mouse. Acta Vitaminol Enzymol. 1975;29(1-6):158-60. [1244085 ]
- Manuelpillai U, Nicholls T, Wallace EM, Phillips DJ, Guillemin G, Walker D: Increased mRNA expression of kynurenine pathway enzymes in human placentae exposed to bacterial endotoxin. Adv Exp Med Biol. 2003;527:85-9. [15206719 ]
- Greengard O: Relationship between urinary excretion of kynurenine and liver tryptophan oxygenase activity. Am J Clin Nutr. 1971 Jun;24(6):709-11. [5581008 ]
- Heinmets F: Computer simulation and analysis of tryptophan metabolism via kynurenine pathway in liver. Comput Biol Med. 1974 Sep;1(4):323-36. [4600391 ]
- Okuno E, Nakamura M, Schwarcz R: Two kynurenine aminotransferases in human brain. Brain Res. 1991 Mar 1;542(2):307-12. [2029638 ]
- Altman K, Greengard O: Correlation of kynurenine excretion with liver tryptophan pyrrolase levels in disease and after hydrocortisone induction. J Clin Invest. 1966 Oct;45(10):1527-34. [5925511 ]
- Pearson SJ, Meldrum A, Reynolds GP: An investigation of the activities of 3-hydroxykynureninase and kynurenine aminotransferase in the brain in Huntington's disease. J Neural Transm Gen Sect. 1995;102(1):67-73. [8785025 ]
- Hartai Z, Klivenyi P, Janaky T, Penke B, Dux L, Vecsei L: Kynurenine metabolism in plasma and in red blood cells in Parkinson's disease. J Neurol Sci. 2005 Dec 15;239(1):31-5. Epub 2005 Aug 15. [16099471 ]
|
|---|