|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110616 |
|---|
|
Identification |
|---|
| Name: |
D-gluconate |
|---|
| Description: | A gluconate having D-configuration. |
|---|
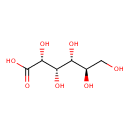
|
Structure |
|
|---|
| Synonyms: | -
D-gluconic acid
-
dextronic acid
-
maltonic acid
|
|---|
|
Chemical Formula: |
C6H11O7
|
|---|
| Average Molecular Weight: |
195.15 |
|---|
| Monoisotopic Molecular
Weight: |
196.0583027399 |
|---|
| InChI Key: |
RGHNJXZEOKUKBD-SQOUGZDYSA-M |
|---|
| InChI: |
InChI=1S/C6H12O7/c7-1-2(8)3(9)4(10)5(11)6(12)13/h2-5,7-11H,1H2,(H,12,13)/p-1/t2-,3-,4+,5-/m1/s1 |
|---|
| CAS
number: |
526-95-4 |
|---|
| IUPAC Name: | D-gluconate |
|---|
|
Traditional IUPAC Name: |
gluconate |
|---|
| SMILES: | C(O)C(O)C(O)C(O)C(O)C(=O)[O-] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as medium-chain hydroxy acids and derivatives. These are hydroxy acids with a 6 to 12 carbon atoms long side chain. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
|
Class |
Hydroxy acids and derivatives |
|---|
| Sub Class | Medium-chain hydroxy acids and derivatives |
|---|
|
Direct Parent |
Medium-chain hydroxy acids and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Medium-chain hydroxy acid
- Medium-chain fatty acid
- Beta-hydroxy acid
- Hydroxy fatty acid
- Fatty acyl
- Fatty acid
- Monosaccharide
- Secondary alcohol
- Carboxylic acid derivative
- Carboxylic acid
- Polyol
- Monocarboxylic acid or derivatives
- Alcohol
- Organic oxygen compound
- Organooxygen compound
- Primary alcohol
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Organic anion
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
- a small molecule (L-IDONATE)
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
113 - 118 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 113 - 118 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 316 mg/mL at 25 °C | MERCK INDEX (1996) | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) | splash10-0002-0932000000-202af87cea2d1f7184ab | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) | splash10-005a-0920000000-2308d9356bc5bb01420e | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) | splash10-014j-0950000000-61ab7adf15e353df4ba2 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (6 TMS) | splash10-0le9-1964000000-5eb7d6777170e1ad5fa0 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS | Not Available |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-06vj-1900000000-78e4a5d92be678c068e4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-03ds-5900000000-f5d8284baa473ce9d77f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-014i-9500000000-7c5b20eef98d0e3d6cbb | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-0002-0900000000-b2632ca9154cc5e44438 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-002b-5900000000-4a3066f9dfd6653682fb | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-004i-9000000000-50f63dfd017a8380a47c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative | splash10-056r-9000000000-91ccf7c8949c1c9d852a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Negative | splash10-0a6r-9000000000-6e40f1cebd8b460016b3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,1H] 2D NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Castrillo JI, Zeef LA, Hoyle DC, Zhang N, Hayes A, Gardner DC, Cornell MJ, Petty J, Hakes L, Wardleworth L, Rash B, Brown M, Dunn WB, Broadhurst D, O'Donoghue K, Hester SS, Dunkley TP, Hart SR, Swainston N, Li P, Gaskell SJ, Paton NW, Lilley KS, Kell DB, Oliver SG (2007)Growth control of the eukaryote cell: a systems biology study in yeast. Journal of biology 6, Pubmed: 17439666
|
|---|
| Synthesis Reference: |
Anastassiadis, Savas; Morgunov, Igor G. Gluconic acid production. Recent Patents on Biotechnology (2007), 1(2), 167-180. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|