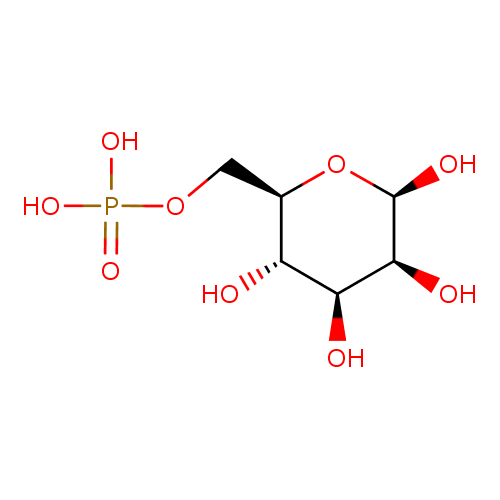
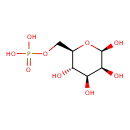| References: |
- Tiede S, Muschol N, Reutter G, Cantz M, Ullrich K, Braulke T: Missense mutations in N-acetylglucosamine-1-phosphotransferase alpha/beta subunit gene in a patient with mucolipidosis III and a mild clinical phenotype. Am J Med Genet A. 2005 Sep 1;137(3):235-40. [16094673 ]
- Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. [19212411 ]
- DeRossi C, Bode L, Eklund EA, Zhang F, Davis JA, Westphal V, Wang L, Borowsky AD, Freeze HH: Ablation of mouse phosphomannose isomerase (Mpi) causes mannose 6-phosphate accumulation, toxicity, and embryonic lethality. J Biol Chem. 2006 Mar 3;281(9):5916-27. Epub 2005 Dec 8. [16339137 ]
- Beljaars L, Molema G, Weert B, Bonnema H, Olinga P, Groothuis GM, Meijer DK, Poelstra K: Albumin modified with mannose 6-phosphate: A potential carrier for selective delivery of antifibrotic drugs to rat and human hepatic stellate cells. Hepatology. 1999 May;29(5):1486-93. [10216133 ]
- van der Ploeg AT, van der Kraaij AM, Willemsen R, Kroos MA, Loonen MC, Koster JF, Reuser AJ: Rat heart perfusion as model system for enzyme replacement therapy in glycogenosis type II. Pediatr Res. 1990 Oct;28(4):344-7. [2235132 ]
- Yatziv S, Barfi G, Newburg DS: Lysosomal hydrolases in blood-derived macrophages of patients with I-cell disease. J Lab Clin Med. 1986 Oct;108(4):365-8. [3093618 ]
- Puolakkainen M, Kuo CC, Campbell LA: Chlamydia pneumoniae uses the mannose 6-phosphate/insulin-like growth factor 2 receptor for infection of endothelial cells. Infect Immun. 2005 Aug;73(8):4620-5. [16040974 ]
- Holtta-Vuori M, Maatta J, Ullrich O, Kuismanen E, Ikonen E: Mobilization of late-endosomal cholesterol is inhibited by Rab guanine nucleotide dissociation inhibitor. Curr Biol. 2000 Jan 27;10(2):95-8. [10662671 ]
- Harper J, Burns JL, Foulstone EJ, Pignatelli M, Zaina S, Hassan AB: Soluble IGF2 receptor rescues Apc(Min/+) intestinal adenoma progression induced by Igf2 loss of imprinting. Cancer Res. 2006 Feb 15;66(4):1940-8. [16488992 ]
- Saris JJ, Derkx FH, De Bruin RJ, Dekkers DH, Lamers JM, Saxena PR, Schalekamp MA, Jan Danser AH: High-affinity prorenin binding to cardiac man-6-P/IGF-II receptors precedes proteolytic activation to renin. Am J Physiol Heart Circ Physiol. 2001 Apr;280(4):H1706-15. [11247783 ]
- Bird CH, Sun J, Ung K, Karambalis D, Whisstock JC, Trapani JA, Bird PI: Cationic sites on granzyme B contribute to cytotoxicity by promoting its uptake into target cells. Mol Cell Biol. 2005 Sep;25(17):7854-67. [16107729 ]
- Adrian JE, Poelstra K, Scherphof GL, Molema G, Meijer DK, Reker-Smit C, Morselt HW, Kamps JA: Interaction of targeted liposomes with primary cultured hepatic stellate cells: Involvement of multiple receptor systems. J Hepatol. 2006 Mar;44(3):560-7. Epub 2005 Oct 19. [16368158 ]
- Ullrich K, Basner R, Gieselmann V, Von Figura K: Recognition of human urine alpha-N-acetylglucosaminidase by rat hepatocytes. Involvement of receptors specific for galactose, mannose 6-phosphate and mannose. Biochem J. 1979 May 15;180(2):413-9. [114170 ]
- Davis JA, Wu XH, Wang L, DeRossi C, Westphal V, Wu R, Alton G, Srikrishna G, Freeze HH: Molecular cloning, gene organization, and expression of mouse Mpi encoding phosphomannose isomerase. Glycobiology. 2002 Jul;12(7):435-42. [12122025 ]
- Kaplan A, Fischer D, Achord D, Sly W: Phosphohexosyl recognition is a general characteristic of pinocytosis of lysosomal glycosidases by human fibroblasts. J Clin Invest. 1977 Nov;60(5):1088-93. [908752 ]
- Lobel P, Dahms NM, Breitmeyer J, Chirgwin JM, Kornfeld S: Cloning of the bovine 215-kDa cation-independent mannose 6-phosphate receptor. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2233-7. [2951738 ]
- Maguchi S, Taniguchi N, Makita A: Elevated activity and increased mannose-6-phosphate in the carbohydrate moiety of cathepsin D from human hepatoma. Cancer Res. 1988 Jan 15;48(2):362-7. [2825973 ]
- Sleat DE, Wang Y, Sohar I, Lackland H, Li Y, Li H, Zheng H, Lobel P: Identification and validation of mannose 6-phosphate glycoproteins in human plasma reveal a wide range of lysosomal and non-lysosomal proteins. Mol Cell Proteomics. 2006 Oct;5(10):1942-56. Epub 2006 May 17. [16709564 ]
- Sleat DE, Sohar I, Lackland H, Majercak J, Lobel P: Rat brain contains high levels of mannose-6-phosphorylated glycoproteins including lysosomal enzymes and palmitoyl-protein thioesterase, an enzyme implicated in infantile neuronal lipofuscinosis. J Biol Chem. 1996 Aug 9;271(32):19191-8. [8702598 ]
|
|---|