|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110584 |
|---|
|
Identification |
|---|
| Name: |
fructose 1,6-bisphosphate |
|---|
| Description: | A D-fructofuranose 1,6-bisphosphate(4−) that is the conjugate base of β-D-fructofuranose 1,6-bisphosphate. |
|---|
|
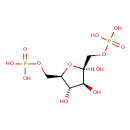
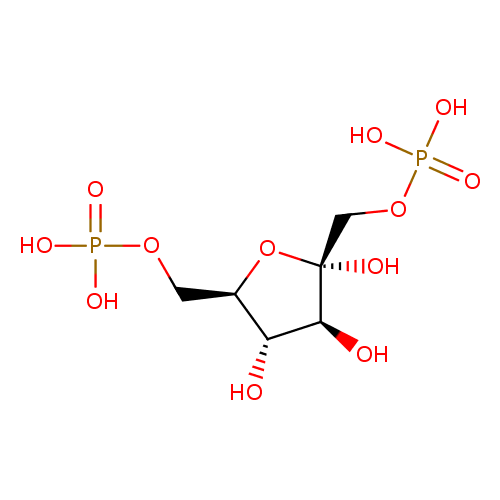
Structure |
|
|---|
| Synonyms: | -
fructose 1,6-biphosphate
-
fructose 1,6-diphosphate
-
β-D-fructose 1,6-diphosphate
-
D-fructose 1,6-diphosphate
-
D-fructos 1,6-bisphosphate
-
fructose 1,6-bisphosphate
-
FBP
|
|---|
|
Chemical Formula: |
C6H10O12P2
|
|---|
| Average Molecular Weight: |
336.08 |
|---|
| Monoisotopic Molecular
Weight: |
339.9960489346 |
|---|
| InChI Key: |
RNBGYGVWRKECFJ-ARQDHWQXSA-J |
|---|
| InChI: |
InChI=1S/C6H14O12P2/c7-4-3(1-16-19(10,11)12)18-6(9,5(4)8)2-17-20(13,14)15/h3-5,7-9H,1-2H2,(H2,10,11,12)(H2,13,14,15)/p-4/t3-,4-,5+,6-/m1/s1 |
|---|
| CAS
number: |
488-69-7 |
|---|
| IUPAC Name: | β-D-fructofuranose 1,6-bisphosphate |
|---|
|
Traditional IUPAC Name: |
fructose 1,6-bisphosphate |
|---|
| SMILES: | C(C1(C(C(C(O1)(COP(=O)([O-])[O-])O)O)O))OP([O-])([O-])=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as hexose phosphates. These are carbohydrate derivatives containing a hexose substituted by one or more phosphate groups. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic oxygen compounds |
|---|
| Sub Class | Organooxygen compounds |
|---|
|
Direct Parent |
Hexose phosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Hexose phosphate
- Pentose phosphate
- Pentose-5-phosphate
- C-glycosyl compound
- Glycosyl compound
- Monosaccharide phosphate
- Pentose monosaccharide
- Monoalkyl phosphate
- Organic phosphoric acid derivative
- Alkyl phosphate
- Phosphoric acid ester
- Oxolane
- 1,2-diol
- Hemiacetal
- Secondary alcohol
- Organoheterocyclic compound
- Oxacycle
- Polyol
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -4 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 94 mg/mL | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Nakayama Y, Kinoshita A, Tomita M: Dynamic simulation of red blood cell metabolism and its application to the analysis of a pathological condition. Theor Biol Med Model. 2005 May 9;2(1):18. [15882454 ]
- Nakai A, Shigematsu Y, Liu YY, Kikawa Y, Sudo M: Urinary sugar phosphates and related organic acids in fructose-1,6-diphosphatase deficiency. J Inherit Metab Dis. 1993;16(2):408-14. [8412001 ]
- Riedel BJ, Gal J, Ellis G, Marangos PJ, Fox AW, Royston D: Myocardial protection using fructose-1,6-diphosphate during coronary artery bypass graft surgery: a randomized, placebo-controlled clinical trial. Anesth Analg. 2004 Jan;98(1):20-9, table of contents. [14693576 ]
- Acan NL, Ozer N: Modification of human erythrocyte pyruvate kinase by an active site-directed reagent: bromopyruvate. J Enzyme Inhib. 2001 Nov;16(5):457-64. [11916152 ]
- Ahn SM, Yoon HY, Lee BG, Park KC, Chung JH, Moon CH, Lee SH: Fructose-1,6-diphosphate attenuates prostaglandin E2 production and cyclo-oxygenase-2 expression in UVB-irradiated HaCaT keratinocytes. Br J Pharmacol. 2002 Oct;137(4):497-503. [12359631 ]
- Norne JE, Lilja H, Lindman B, Einarsson R, Zeppezauer M: Pt(CN)2-4 and Au(CN)-2: potential general probes for anion-binding sites of proteins. 35Cl and 81Br nuclear-magnetic-resonance studies. Eur J Biochem. 1975 Nov 15;59(2):463-73. [1204623 ]
|
|---|
| Synthesis Reference: |
Yan, Weiqun. Method for producing fructose-1,6-diphosphate (FDP). Faming Zhuanli Shenqing Gongkai Shuomingshu (2005), 7 pp. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|