|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110577 |
|---|
|
Identification |
|---|
| Name: |
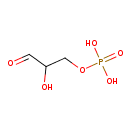
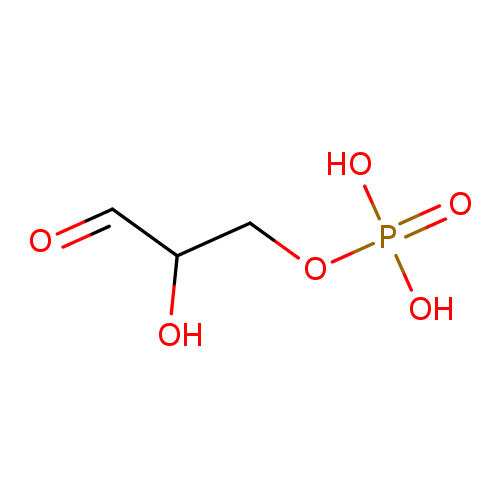
D-glyceraldehyde 3-phosphate |
|---|
| Description: | Dianion of D-glyceraldehyde 3-phosphate arising from deprotonation of both OH groups of the phosphate. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
3-phosphoglyceraldehyde
-
D-glyceraldehyde-3-P
-
glyceraldehyde 3-phosphate
-
GAP
-
glyceraldehyde-phosphate
-
glyceraldehyde-P
-
glyceraldehyde-3-P
-
(2R)-2-hydroxy-3-(phosphonooxy)-propanal
-
triose phosphate
|
|---|
|
Chemical Formula: |
C3H5O6P
|
|---|
| Average Molecular Weight: |
168.04 |
|---|
| Monoisotopic Molecular
Weight: |
169.9980244673 |
|---|
| InChI Key: |
LXJXRIRHZLFYRP-VKHMYHEASA-L |
|---|
| InChI: |
InChI=1S/C3H7O6P/c4-1-3(5)2-9-10(6,7)8/h1,3,5H,2H2,(H2,6,7,8)/p-2/t3-/m0/s1 |
|---|
| CAS
number: |
142-10-9 |
|---|
| IUPAC Name: | (2R)-2-hydroxy-3-oxopropyl phosphate |
|---|
|
Traditional IUPAC Name: |
glyceraldehyde 3 phosphate |
|---|
| SMILES: | [CH](=O)C(O)COP(=O)([O-])[O-] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as glyceraldehyde-3-phosphates. These are compounds containing a glyceraldehyde substituted at position O3 by a phosphate group. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic oxygen compounds |
|---|
| Sub Class | Organooxygen compounds |
|---|
|
Direct Parent |
Glyceraldehyde-3-phosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Glyceraldehyde-3-phosphate
- Monoalkyl phosphate
- Alkyl phosphate
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Alpha-hydroxyaldehyde
- Secondary alcohol
- Organic oxide
- Hydrocarbon derivative
- Carbonyl group
- Aldehyde
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Nakayama Y, Kinoshita A, Tomita M: Dynamic simulation of red blood cell metabolism and its application to the analysis of a pathological condition. Theor Biol Med Model. 2005 May 9;2(1):18. [15882454 ]
- Yamamoto T, Moriwaki Y, Takahashi S, Ohata H, Nakano T, Yamakita J, Higashino K: Effect of glucagon on the xylitol-induced increase in the plasma concentration and urinary excretion of purine bases. Metabolism. 1996 Nov;45(11):1354-9. [8931639 ]
- Choei H, Sasaki N, Takeuchi M, Yoshida T, Ukai W, Yamagishi S, Kikuchi S, Saito T: Glyceraldehyde-derived advanced glycation end products in Alzheimer's disease. Acta Neuropathol (Berl). 2004 Sep;108(3):189-93. Epub 2004 Jun 17. [15221334 ]
- Modun B, Morrissey J, Williams P: The staphylococcal transferrin receptor: a glycolytic enzyme with novel functions. Trends Microbiol. 2000 May;8(5):231-7. [10785640 ]
- Zhang J, Jung K, Lein M, Kristiansen G, Rudolph B, Hauptmann S, Schnorr D, Loening SA, Lichtinghagen R: Differential expression of matrix metalloproteinases and their tissue inhibitors in human primary cultured prostatic cells and malignant prostate cell lines. Prostate. 2002 Jan 1;50(1):38-45. [11757034 ]
- Kogler H, Schott P, Toischer K, Milting H, Van PN, Kohlhaas M, Grebe C, Kassner A, Domeier E, Teucher N, Seidler T, Knoll R, Maier LS, El-Banayosy A, Korfer R, Hasenfuss G: Relevance of brain natriuretic peptide in preload-dependent regulation of cardiac sarcoplasmic reticulum Ca2+ ATPase expression. Circulation. 2006 Jun 13;113(23):2724-32. Epub 2006 Jun 5. [16754798 ]
- Yang Y, Hou Y, Wang CL, Ji SJ: Renal expression of epidermal growth factor and transforming growth factor-beta1 in children with congenital hydronephrosis. Urology. 2006 Apr;67(4):817-21; discussion 821-2. [16618565 ]
- Harper LV, Hilton AC, Jones AF: RT-PCR for the pseudogene-free amplification of the glyceraldehyde-3-phosphate dehydrogenase gene (gapd). Mol Cell Probes. 2003 Oct;17(5):261-5. [14580401 ]
|
|---|
| Synthesis Reference: |
Ballou, Clinton E.; Fischer, Hermann O. L. The synthesis of D-glyceraldehyde 3-phosphate. Journal of the American Chemical Society (1955), 77 3329-31. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|