|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110554 |
|---|
|
Identification |
|---|
| Name: |
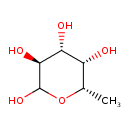
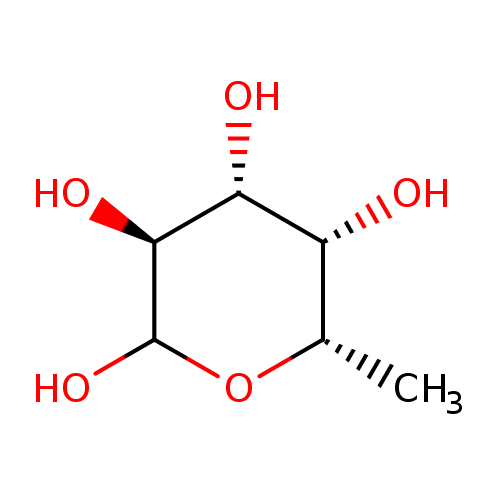
α-L-fucopyranose |
|---|
| Description: | An L-fucopyranose having α-configuration at the anomeric centre. |
|---|
|
Structure |
|
|---|
| Synonyms: | |
|---|
|
Chemical Formula: |
C6H12O5
|
|---|
| Average Molecular Weight: |
164.16 |
|---|
| Monoisotopic Molecular
Weight: |
164.0684734957 |
|---|
| InChI Key: |
SHZGCJCMOBCMKK-SXUWKVJYSA-N |
|---|
| InChI: |
InChI=1S/C6H12O5/c1-2-3(7)4(8)5(9)6(10)11-2/h2-10H,1H3/t2-,3+,4+,5-,6+/m0/s1 |
|---|
| CAS
number: |
2438-80-4 |
|---|
| IUPAC Name: | 6-deoxy-α-L-galactopyranose |
|---|
|
Traditional IUPAC Name: |
L-fucose |
|---|
| SMILES: | CC1(OC(C(C(C1O)O)O)O) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as hexoses. These are monosaccharides in which the sugar unit is a is a six-carbon containing moeity. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic oxygen compounds |
|---|
| Sub Class | Organooxygen compounds |
|---|
|
Direct Parent |
Hexoses |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Hexose monosaccharide
- Oxane
- Secondary alcohol
- Hemiacetal
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Hydrocarbon derivative
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
140 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 140 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 985 mg/mL | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- Fructose and Mannose Degradation pae00051
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Schneider C, Smith DF, Cummings RD, Boligan KF, Hamilton RG, Bochner BS, Miescher S, Simon HU, Pashov A, Vassilev T, von Gunten S (2015)The human IgG anti-carbohydrate repertoire exhibits a universal architecture and contains specificity for microbial attachment sites. Science translational medicine 7, Pubmed: 25568069
- von Gunten S, Smith DF, Cummings RD, Riedel S, Miescher S, Schaub A, Hamilton RG, Bochner BS (2009)Intravenous immunoglobulin contains a broad repertoire of anticarbohydrate antibodies that is not restricted to the IgG2 subclass. The Journal of allergy and clinical immunology 123, Pubmed: 19443021
|
|---|
| Synthesis Reference: |
Xu, Zuhong; Zhao, Zengqin; Zhang, Quanbin; Niu, Xizhen; Zhang, Hong; Li, Zhien. Method for preparing L-fucose from laminaria japonica. Faming Zhuanli Shenqing Gongkai Shuomingshu (2005), 8 pp. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|