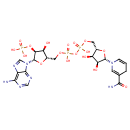
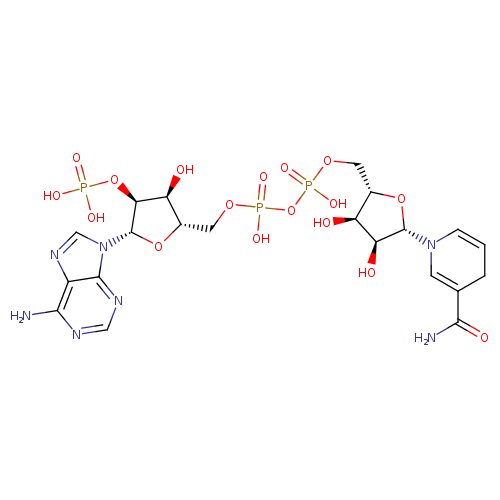
NADPH (PAMDB110539)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB110539 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | NADPH | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Tetraanion of NADPH arising from deprotonation of the diphosphate and phosphate OH groups; major species at pH 7.3. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C21H26N7O17P3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 741.39 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 745.0911021051 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | ACFIXJIJDZMPPO-NNYOXOHSSA-J | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C21H30N7O17P3/c22-17-12-19(25-7-24-17)28(8-26-12)21-16(44-46(33,34)35)14(30)11(43-21)6-41-48(38,39)45-47(36,37)40-5-10-13(29)15(31)20(42-10)27-3-1-2-9(4-27)18(23)32/h1,3-4,7-8,10-11,13-16,20-21,29-31H,2,5-6H2,(H2,23,32)(H,36,37)(H,38,39)(H2,22,24,25)(H2,33,34,35)/p-4/t10-,11-,13-,14-,15-,16-,20-,21-/m1/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 53-57-6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | 2'- | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | [(2S,3S,4S,5S)-2-(6-aminopurin-9-yl)-5-{[({[(2S,3R,4S,5S)-5-(3-carbamoyl-4H-pyridin-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy(hydroxy)phosphoryl}oxy(hydroxy)phosphoryl)oxy]methyl}-4-hydroxyoxolan-3-yl]oxyphosphonic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | C5(N(C1(OC(C(C1O)O)COP(OP(OCC4(C(C(C(N3(C2(=C(C(=NC=N2)N)N=C3)))O4)OP([O-])([O-])=O)O))([O-])=O)(=O)[O-]))C=C(CC=5)C(=O)N) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of chemical entities known as (5'->5')-dinucleotides. These are dinucleotides where the two bases are connected via a (5'->5')-phosphodiester linkage. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Chemical entities | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Nucleosides, nucleotides, and analogues | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | (5'->5')-dinucleotides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | (5'->5')-dinucleotides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Acetaldehyde + NADP+ + Water → acetate + NADPH + Hydrogen ion 2-trans, 4-trans-undecadienoyl-CoA + NADPH + Hydrogen ion → 3-trans-undecenoyl-CoA + NADP+ phenylacetyl-CoA + Oxygen + NADPH + Hydrogen ion → 2-(1,2-epoxy-1,2-dihydrophenyl)acetyl-CoA + NADP+ + Water preQ1 + NADP+ → 7-Cyano-7-carbaguanine + NADPH + Hydrogen ion protochlorophyllide a + NADP+ → 2,4-divinyl protochlorophyllide a + NADPH + Hydrogen ion LysW-L-glutamate-5-semialdehyde + NADP+ + phosphate → LysW-L-glutamate-5-phosphate + NADPH + Hydrogen ion magnesium-protoporphyrin IX 13-monomethyl ester + Water + NADP+ → 131-hydroxy-magnesium-protoporphyrin IX 13-monomethyl ester + NADPH + Hydrogen ion salicylate + L-Cysteine + S-Adenosylmethionine + NADPH → pyochelin + S-Adenosylhomocysteine + NADP+ + Water NADP+ → NADPH + Hydrogen ion tRNA-Dihydrouridines + NADP+ → tRNA-uridines + NADPH + Hydrogen ion N,N'-dimethyl-p-phenylenediamine + aniline + NADP+ → 4-dimethylaminophenylazobenzene + NADPH + Hydrogen ion More...2-trans, 4-cis-undecadienoyl-CoA + NADPH + Hydrogen ion → 3-trans-undecenoyl-CoA + NADP+ Hydrogen ion + methyl-1,4-benzoquinone + NADPH → methyl-1,4-benzoquinol + NADP+ 131-hydroxy-magnesium-protoporphyrin IX 13-monomethyl ester + NADP+ → 131-oxo-magnesium-protoporphyrin IX 13-monomethyl ester + NADPH + Hydrogen ion D-glyceraldehyde 3-phosphate + NADP+ + Water → Hydrogen ion + 3-phospho-D-glycerate + NADPH NADPH + Hydrogen ion → NADP+ + Water D-glyceraldehyde 3-phosphate + phosphate + NADP+ → 1,3-bisphospho-D-glycerate + NADPH + Hydrogen ion 2-trans,4-trans-tetradecadienoyl-CoA + NADPH + Hydrogen ion → 3-trans-tetradecenoyl-CoA + NADP+ magnesium-protoporphyrin IX 13-monomethyl ester + Oxygen + NADPH + Hydrogen ion → 131-hydroxy-magnesium-protoporphyrin IX 13-monomethyl ester + NADP+ + Water NADPH + Hydrogen ion → NADP+ + Water + Carbon dioxide 5-hydroxy-3-[(3aS,4S,5R,7aS)-7a-methyl-1,5-dioxo-octahydro-1H-inden-4-yl]propanoyl-CoA + NADP+ → 3-[(3aS,4S,7aS)-7a-methyl-1,5-dioxo-octahydro-1H-inden-4-yl]propanoyl-CoA + NADPH + Hydrogen ion (R)-2,3-dihydroxy-3-methylbutanoate + NADP+ → (S)-2-acetolactate + NADPH + Hydrogen ion (S)-nicotine + NADPH + Oxygen → nicotine-1'-N-oxide + NADP+ + Water Nitrite + Water + NADP+ → Hydrogen ion + Nitrate + NADPH (S)-3-hydroxybutanoyl-CoA + NADP+ → acetoacetyl-CoA + NADPH + Hydrogen ion apo-4'-lycopenal + NAD-P-OR-NOP + Water → apo-4'-lycopenoate + Hydrogen ion + NADPH benzaldehyde + Water + NADP+ → benzoate + NADPH + Hydrogen ion TETRADEHYDROACYL-COA + NADPH → Trans-3-enoyl-CoAs + NADP+ 131-hydroxy-magnesium-protoporphyrin IX 13-monomethyl ester + NADPH + Hydrogen ion + Oxygen → 131-oxo-magnesium-protoporphyrin IX 13-monomethyl ester + NADP+ + Water Glucopyranose + NADP+ → Gluconolactone + NADPH + Hydrogen ion TRANS-D2-ENOYL-COA + NADP+ → TETRADEHYDROACYL-COA + NADPH + Hydrogen ion NADPH → NADP+ selenodiglutathione + NADPH → glutathioselenol + glutathione + NADP+ (R)-3-hydroxybutanoyl-CoA + NADP+ → acetoacetyl-CoA + NADPH + Hydrogen ion 2-keto-D-gluconate + NADP+ → Hydrogen ion + NADPH + 2,5-Diketo-D-gluconate NADP+ + NADH + Hydrogen ion → NADPH + NAD+ + Hydrogen ion 3-oxo-5,6-didehydrosuberyl-CoA semialdehyde + NADP+ + Water → 3-oxo-5,6-didehydrosuberyl-CoA + NADPH + Hydrogen ion 131-oxo-magnesium-protoporphyrin IX 13-monomethyl ester + Oxygen + NADPH → 2,4-divinyl protochlorophyllide a + NADP+ + Water 2-trans-4-cis-dienoyl-CoAs + NADPH → Trans-3-enoyl-CoAs + NADP+ + Hydrogen ion reduced riboflavin + NADP+ → Hydrogen ion + riboflavin + NADPH 1-oleoyl-2-lyso-glycerone phosphate + NADPH + Hydrogen ion → 1-oleyl-2-lyso-phosphatidate + NADP+ L-ornithine + Oxygen + NADPH → N5-hydroxy-L-ornithine + Water + NADP+ Aldehydes + NADP+ + Water → Carboxylates + NADPH + Hydrogen ion chlorophyllide a + NADP+ → Hydrogen ion + protochlorophyllide a + NADPH glutathioselenol + NADPH + Hydrogen ion → Hydrogen selenide + glutathione + NADP+ 131-oxo-magnesium-protoporphyrin IX 13-monomethyl ester + NADP+ → 2,4-divinyl protochlorophyllide a + NADPH + Hydrogen ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Seelbach, Karsten; Riebel, Bettina; Hummel, Werner; Kula, Maria-Regina; Tishkov, Vladimir I.; Egorov, Alexey M.; Wandrey, Christian; Kragl, Udo. A novel, efficient regenerating method of NADPH using a new formate dehydrogenase. Tetrahedron Letters (1996), 37(9), 1377-80. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||