|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110536 |
|---|
|
Identification |
|---|
| Name: |
dTDP |
|---|
| Description: | An organophosphate oxoanion arising from deprotonation of the diphosphate OH groups of thymidine 5'-diphosphate; major species at pH 7.3. |
|---|
|
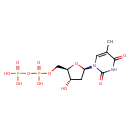
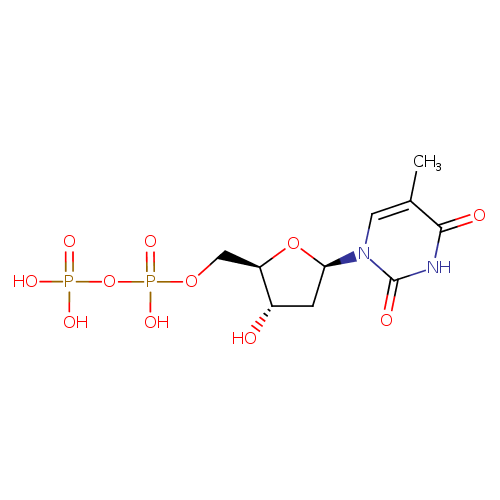
Structure |
|
|---|
| Synonyms: | -
TDP
-
thymidine 5'-(trihydrogen diphosphate) (9CI)
-
deoxy-TDP
-
thymidine-5'-diphosphate
-
thymidine-diphosphate
|
|---|
|
Chemical Formula: |
C10H13N2O11P2
|
|---|
| Average Molecular Weight: |
399.17 |
|---|
| Monoisotopic Molecular
Weight: |
402.0229323871 |
|---|
| InChI Key: |
UJLXYODCHAELLY-XLPZGREQSA-K |
|---|
| InChI: |
InChI=1S/C10H16N2O11P2/c1-5-3-12(10(15)11-9(5)14)8-2-6(13)7(22-8)4-21-25(19,20)23-24(16,17)18/h3,6-8,13H,2,4H2,1H3,(H,19,20)(H,11,14,15)(H2,16,17,18)/p-3/t6-,7+,8+/m0/s1 |
|---|
| CAS
number: |
491-97-4 |
|---|
| IUPAC Name: | 5'-O-[(phosphonatooxy)phosphinato]thymidine |
|---|
|
Traditional IUPAC Name: |
dTDP |
|---|
| SMILES: | CC1(=CN(C(=O)NC(=O)1)C2(CC(O)C(COP(=O)([O-])OP(=O)([O-])[O-])O2)) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as pyrimidine 2'-deoxyribonucleoside diphosphates. These are pyrimidine nucleotides with a diphosphate group linked to the ribose moiety lacking a hydroxyl group at position 2. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Nucleosides, nucleotides, and analogues |
|---|
| Sub Class | Pyrimidine nucleotides |
|---|
|
Direct Parent |
Pyrimidine 2'-deoxyribonucleoside diphosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Pyrimidine 2'-deoxyribonucleoside diphosphate
- Organic pyrophosphate
- Monoalkyl phosphate
- Pyrimidone
- Hydropyrimidine
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Pyrimidine
- Alkyl phosphate
- Oxolane
- Heteroaromatic compound
- Vinylogous amide
- Lactam
- Urea
- Secondary alcohol
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Alcohol
- Organic nitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -3 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Bialkowski K, Kasprzak KS: Inhibition of 8-oxo-2'-deoxyguanosine 5'-triphosphate pyrophosphohydrolase (8-oxo-dGTPase) activity of the antimutagenic human MTH1 protein by nucleoside 5'-diphosphates. Free Radic Biol Med. 2003 Sep 15;35(6):595-602. [12957652 ]
- Dahlmann N: Human serum thymidine triphosphate nucleotidohydrolase: purification and properties of a new enzyme. Biochemistry. 1982 Dec 21;21(26):6634-9. [6297538 ]
- Xu Y, Singh KV, Qin X, Murray BE, Weinstock GM: Analysis of a gene cluster of Enterococcus faecalis involved in polysaccharide biosynthesis. Infect Immun. 2000 Feb;68(2):815-23. [10639451 ]
- Vallon O: New sequence motifs in flavoproteins: evidence for common ancestry and tools to predict structure. Proteins. 2000 Jan 1;38(1):95-114. [10651042 ]
- Sheu SJ, Wu SN: Mechanism of inhibitory actions of oxidizing agents on calcium-activated potassium current in cultured pigment epithelial cells of the human retina. Invest Ophthalmol Vis Sci. 2003 Mar;44(3):1237-44. [12601054 ]
- Costantini P, Belzacq AS, Vieira HL, Larochette N, de Pablo MA, Zamzami N, Susin SA, Brenner C, Kroemer G: Oxidation of a critical thiol residue of the adenine nucleotide translocator enforces Bcl-2-independent permeability transition pore opening and apoptosis. Oncogene. 2000 Jan 13;19(2):307-14. [10645010 ]
- Ramaswamy SV, Amin AG, Goksel S, Stager CE, Dou SJ, El Sahly H, Moghazeh SL, Kreiswirth BN, Musser JM: Molecular genetic analysis of nucleotide polymorphisms associated with ethambutol resistance in human isolates of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2000 Feb;44(2):326-36. [10639358 ]
- Tomioka H: [Prospects for development of new antituberculous drugs] Kekkaku. 2002 Aug;77(8):573-84. [12235850 ]
- Kuo SY, Jiann BP, Lu YC, Chang HT, Chen WC, Huang JK, Jan CR: Thiol oxidation by 2,2'-dithiodipyridine induced calcium mobilization in MG63 human osteosarcoma cells. Life Sci. 2003 Feb 28;72(15):1733-43. [12559394 ]
- Riener CK, Kada G, Gruber HJ: Quick measurement of protein sulfhydryls with Ellman's reagent and with 4,4'-dithiodipyridine. Anal Bioanal Chem. 2002 Jul;373(4-5):266-76. Epub 2002 Jun 6. [12110978 ]
- Ahmed IH, Manning G, Wassenaar TM, Cawthraw S, Newell DG: Identification of genetic differences between two Campylobacter jejuni strains with different colonization potentials. Microbiology. 2002 Apr;148(Pt 4):1203-12. [11932464 ]
- Kuo SY, Ho CM, Chen WC, Jan CR: Sulfhydryl modification by 4,4'-dithiodipyridine induces calcium mobilization in human osteoblast-like cells. Arch Toxicol. 2003 Nov;77(11):630-7. Epub 2003 Aug 20. [12928766 ]
|
|---|
| Synthesis Reference: |
Rupprath, Carsten; Kopp, Maren; Hirtz, Dennis; Mueller, Rolf; Elling, Lothar. An enzyme module system for in situ regeneration of deoxythymidine 5'-diphosphate (dTDP)-activated deoxy sugars. Advanced Synthesis & Catalysis (2007), 349(8+9), 1489-1496. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|