|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110535 |
|---|
|
Identification |
|---|
| Name: |
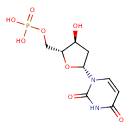
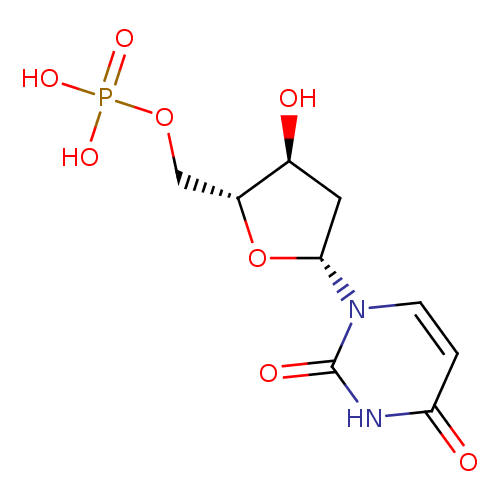
dUMP |
|---|
| Description: | Dianion of deoxyuridine 5'-phosphate arising from deprotonation of the phosphate OH groups; major species at pH 7.3. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
U
-
deoxyurdine-phosphate
-
2'-deoxyuridine 5-monophosphate
-
deoxyuridine-phosphate
-
2'-deoxyuridine 5'-phosphate
-
2'-deoxyuridylic acid
-
2'-deoxy-5'-uridylic acid
|
|---|
|
Chemical Formula: |
C9H11N2O8P
|
|---|
| Average Molecular Weight: |
306.17 |
|---|
| Monoisotopic Molecular
Weight: |
308.0409519145 |
|---|
| InChI Key: |
JSRLJPSBLDHEIO-SHYZEUOFSA-L |
|---|
| InChI: |
InChI=1S/C9H13N2O8P/c12-5-3-8(11-2-1-7(13)10-9(11)14)19-6(5)4-18-20(15,16)17/h1-2,5-6,8,12H,3-4H2,(H,10,13,14)(H2,15,16,17)/p-2/t5-,6+,8+/m0/s1 |
|---|
| CAS
number: |
964-26-1 |
|---|
| IUPAC Name: | 2'-deoxy-5'-O-phosphonatouridine |
|---|
|
Traditional IUPAC Name: |
2'-deoxyuridylic acid |
|---|
| SMILES: | C(OP(=O)([O-])[O-])C1(OC(CC(O)1)N2(C=CC(=O)NC(=O)2)) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as pyrimidine 2'-deoxyribonucleoside monophosphates. These are pyrimidine nucleotides with a monophosphate group linked to the ribose moiety lacking a hydroxyl group at position 2. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Nucleosides, nucleotides, and analogues |
|---|
| Sub Class | Pyrimidine nucleotides |
|---|
|
Direct Parent |
Pyrimidine 2'-deoxyribonucleoside monophosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Pyrimidine 2'-deoxyribonucleoside monophosphate
- Hydroxypyrimidine
- Pyrimidone
- Monoalkyl phosphate
- Hydropyrimidine
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Pyrimidine
- Alkyl phosphate
- Oxolane
- Heteroaromatic compound
- Secondary alcohol
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Alcohol
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS | Not Available |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, N/A (Annotated) | splash10-001i-9100000000-c9b7092bb72f342d372f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, N/A (Annotated) | splash10-001i-9000000000-d2272878a49bc62c1955 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, N/A (Annotated) | splash10-001i-9000000000-69451d809e5517ef09cb | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative | splash10-01ta-9803000000-f0e26cbfb75f3fb7b36e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Richards RG, Sowers LC, Laszlo J, Sedwick WD: The occurrence and consequences of deoxyuridine in DNA. Adv Enzyme Regul. 1984;22:157-85. [6147963 ]
- Krokan HE, Otterlei M, Nilsen H, Kavli B, Skorpen F, Andersen S, Skjelbred C, Akbari M, Aas PA, Slupphaug G: Properties and functions of human uracil-DNA glycosylase from the UNG gene. Prog Nucleic Acid Res Mol Biol. 2001;68:365-86. [11554311 ]
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|