|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110532 |
|---|
|
Identification |
|---|
| Name: |
dCTP |
|---|
| Description: | A 2'-deoxyribonucleoside triphosphate oxoanion arising from deprotonation of the triphosphate OH groups of 2'-deoxycytidine 5'-triphosphate; major species at pH 7.3. |
|---|
|
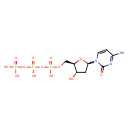
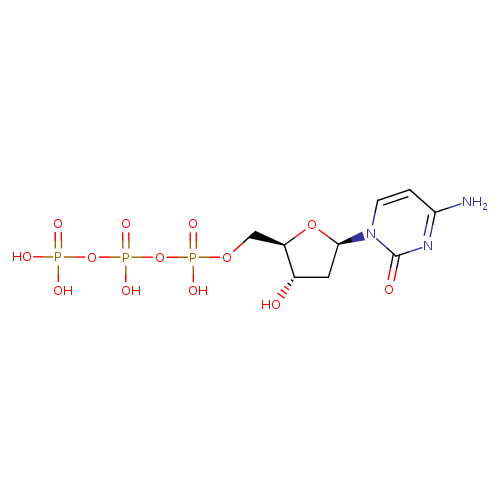
Structure |
|
|---|
| Synonyms: | -
2'-deoxycytidine-5'-triphosphate
-
deoxycytidine-triphosphate
-
deoxy-CTP
|
|---|
|
Chemical Formula: |
C9H12N3O13P3
|
|---|
| Average Molecular Weight: |
463.13 |
|---|
| Monoisotopic Molecular
Weight: |
466.9895971465 |
|---|
| InChI Key: |
RGWHQCVHVJXOKC-SHYZEUOFSA-J |
|---|
| InChI: |
InChI=1S/C9H16N3O13P3/c10-7-1-2-12(9(14)11-7)8-3-5(13)6(23-8)4-22-27(18,19)25-28(20,21)24-26(15,16)17/h1-2,5-6,8,13H,3-4H2,(H,18,19)(H,20,21)(H2,10,11,14)(H2,15,16,17)/p-4/t5-,6+,8+/m0/s1 |
|---|
| CAS
number: |
2056-98-6 |
|---|
| IUPAC Name: | 2'-deoxy-5'-O-({[(phosphonatooxy)phosphinato]oxy}phosphinato)cytidine |
|---|
|
Traditional IUPAC Name: |
dCTP |
|---|
| SMILES: | C(C2(C(CC(N1(C(N=C(C=C1)N)=O))O2)O))OP(OP(OP([O-])([O-])=O)([O-])=O)([O-])=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as pyrimidine 2'-deoxyribonucleoside triphosphates. These are pyrimidine nucleotides with a triphosphate group linked to the ribose moiety lacking a hydroxyl group at position 2. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Nucleosides, nucleotides, and analogues |
|---|
| Sub Class | Pyrimidine nucleotides |
|---|
|
Direct Parent |
Pyrimidine 2'-deoxyribonucleoside triphosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Pyrimidine 2'-deoxyribonucleoside triphosphate
- Hydroxypyrimidine
- Monoalkyl phosphate
- Hydropyrimidine
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Pyrimidine
- Alkyl phosphate
- Oxolane
- Heteroaromatic compound
- Secondary alcohol
- Oxacycle
- Organoheterocyclic compound
- Azacycle
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Alcohol
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -4 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Yamauchi T, Ueda T: A sensitive new method for clinically monitoring cytarabine concentrations at the DNA level in leukemic cells. Biochem Pharmacol. 2005 Jun 15;69(12):1795-803. Epub 2005 Apr 26. [15935150 ]
- Brzezianska E, Zdzieszynska M, Gos R, Lewinski A: [Genetic analysis of rhodopsin and peripherin genes in patients with autosomal dominant retinitis pigmentosa (adRP) in Polish families] Klin Oczna. 2004;106(6):743-8. [15787173 ]
- van 't Wout AB: Gene expression profiling of HIV-1 infection using cDNA microarrays. Methods Mol Biol. 2005;304:455-9. [16061997 ]
- Moriarty TJ, Marie-Egyptienne DT, Autexier C: Regulation of 5' template usage and incorporation of noncognate nucleotides by human telomerase. RNA. 2005 Sep;11(9):1448-60. [16120835 ]
- Choi JY, Guengerich FP: Adduct size limits efficient and error-free bypass across bulky N2-guanine DNA lesions by human DNA polymerase eta. J Mol Biol. 2005 Sep 9;352(1):72-90. [16061253 ]
|
|---|
| Synthesis Reference: |
Hinz M; Gottschling D; Eritja R; Seliger H Synthesis and properties of 2'-deoxycytidine triphosphate carrying c-myc tag sequence. Nucleosides, nucleotides & nucleic acids (2000), 19(10-12), 1543-52. |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|