| InChI: |
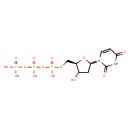
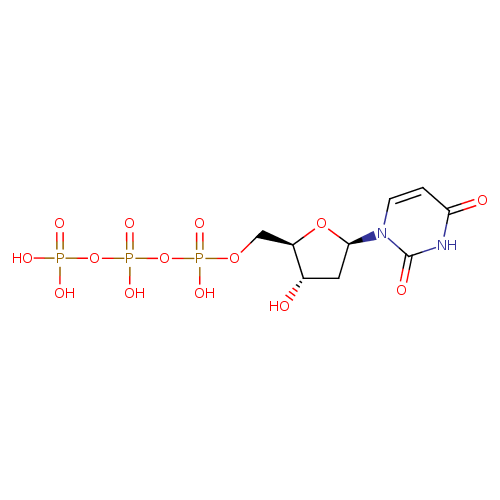
InChI=1S/C9H15N2O14P3/c12-5-3-8(11-2-1-7(13)10-9(11)14)23-6(5)4-22-27(18,19)25-28(20,21)24-26(15,16)17/h1-2,5-6,8,12H,3-4H2,(H,18,19)(H,20,21)(H,10,13,14)(H2,15,16,17)/p-4/t5-,6+,8+/m0/s1 |
|---|
| References: |
- Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. [19212411 ]
- Lee AY, Youm YH, Kim NH, Yang H, Choi WI: Keratinocytes in the depigmented epidermis of vitiligo are more vulnerable to trauma (suction) than keratinocytes in the normally pigmented epidermis, resulting in their apoptosis. Br J Dermatol. 2004 Nov;151(5):995-1003. [15541077 ]
- Oosterhuis GJ, Mulder AB, Kalsbeek-Batenburg E, Lambalk CB, Schoemaker J, Vermes I: Measuring apoptosis in human spermatozoa: a biological assay for semen quality? Fertil Steril. 2000 Aug;74(2):245-50. [10927039 ]
- Ge YF, Huang YF, Zhang GY, Wang XH, Xu JP: Studies on apoptosis of spermatogenic cells in normal fertile men treated with supraphysiological doses of testosterone undecanoate. Asian J Androl. 1999 Sep;1(3):155-8. [11250785 ]
- Gilhar A, Ullmann Y, Karry R, Shalaginov R, Assy B, Serafimovich S, Kalish RS: Ageing of human epidermis: the role of apoptosis, Fas and telomerase. Br J Dermatol. 2004 Jan;150(1):56-63. [14746617 ]
- Igarashi T, Brown CR, Byrum RA, Nishimura Y, Endo Y, Plishka RJ, Buckler C, Buckler-White A, Miller G, Hirsch VM, Martin MA: Rapid and irreversible CD4+ T-cell depletion induced by the highly pathogenic simian/human immunodeficiency virus SHIV(DH12R) is systemic and synchronous. J Virol. 2002 Jan;76(1):379-91. [11739702 ]
- Russell J, O'Donoghue JA, Finn R, Koziorowski J, Ruan S, Humm JL, Ling CC: Iodination of annexin V for imaging apoptosis. J Nucl Med. 2002 May;43(5):671-7. [11994533 ]
- Lamperti C, Naini AB, Lucchini V, Prelle A, Bresolin N, Moggio M, Sciacco M, Kaufmann P, DiMauro S: Muscle coenzyme Q10 level in statin-related myopathy. Arch Neurol. 2005 Nov;62(11):1709-12. [16286544 ]
|
|---|