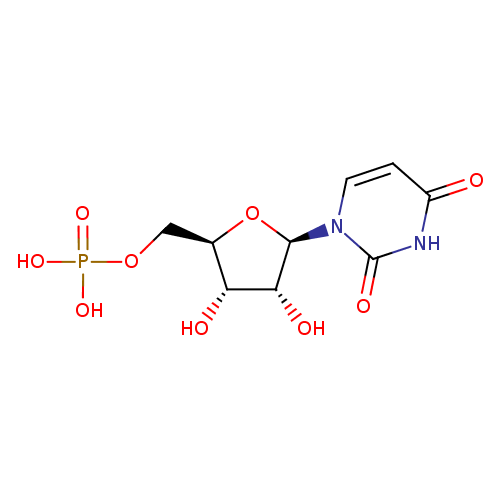
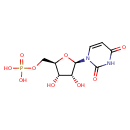UMP (PAMDB110527)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB110527 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | UMP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | A nucleoside 5'-monophosphate(2−) that results from the removal of two protons from the phosphate group of UMP. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C9H11N2O9P | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 322.17 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 324.0358665366 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | DJJCXFVJDGTHFX-XVFCMESISA-L | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C9H13N2O9P/c12-5-1-2-11(9(15)10-5)8-7(14)6(13)4(20-8)3-19-21(16,17)18/h1-2,4,6-8,13-14H,3H2,(H,10,12,15)(H2,16,17,18)/p-2/t4-,6-,7-,8-/m1/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 58-97-9 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | 5'-O-phosphonatouridine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | uridylate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | C(OP(=O)([O-])[O-])C1(OC(C(O)C(O)1)N2(C=CC(=O)NC(=O)2)) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of chemical entities known as pyrimidine ribonucleoside monophosphates. These are pyrimidine ribobucleotides with monophosphate group linked to the ribose moiety. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Chemical entities | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Nucleosides, nucleotides, and analogues | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Pyrimidine nucleotides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Pyrimidine ribonucleoside monophosphates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteromonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | UDP-N-acetyl-α-D-muramoyl-L-alanyl-γ-D-glutamyl-L-lysyl-D-alanine + di-trans,octa-cis-undecaprenyl phosphate → undecaprenyl-diphospho-N-acetylmuramoyl-L-alanyl-γ-D-glutamyl-L-lysyl- D-alanine + UMP UDP-N-acetyl-α-D-muramoyl-L-alanyl-γ-D-glutamyl-L-lysyl-D-alanyl-D-alanine + di-trans,octa-cis-undecaprenyl phosphate → undecaprenyl-diphospho-N-acetylmuramoyl-L-alanyl-γ-D-glutamyl-L-lysyl- D-alanyl-D-alanine + UMP UDP-N-acetyl-α-D-muramoyl-L-alanyl-γ-D-glutamyl-meso-2,6-diaminopimeloyl-D-alanyl-D-alanine + trans,octacis-decaprenyl phosphate → decaprenyl-diphospho-N-acetylmuramoyl-pentapeptide + UMP UTP + Water → UMP + diphosphate + Hydrogen ion UMP + Water → Uridine + phosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Wang Xing; Wang Xiuwen; Yin Mengxin; Xiao Zijun; Ma Cuiqing; Lin Zhixin; Wang Peng George; Xu Ping Production of uridine 5'-monophosphate by Corynebacterium ammoniagenes ATCC 6872 using a statistically improved biocatalytic process. Applied microbiology and biotechnology (2007), 76(2), 321-8. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||