|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110503 |
|---|
|
Identification |
|---|
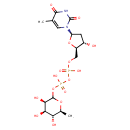
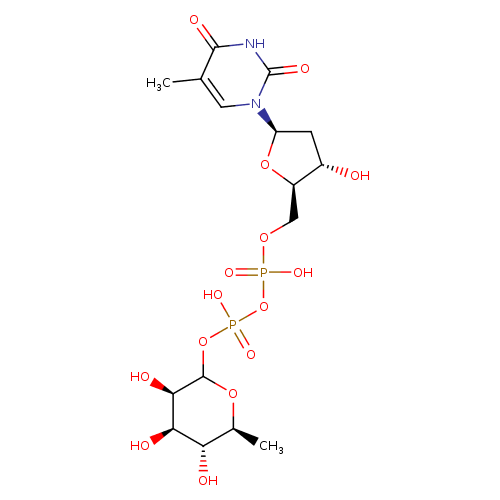
| Name: |
dTDP-β-L-rhamnose |
|---|
| Description: | Dianion of dTDP-6-deoxy-β-L-mannose arising from deprotonation of both free OH groups of the diphosphate. |
|---|
|
Structure |
|
|---|
| Synonyms: | |
|---|
|
Chemical Formula: |
C16H24N2O15P2
|
|---|
| Average Molecular Weight: |
546.32 |
|---|
| Monoisotopic Molecular
Weight: |
548.0808411965 |
|---|
| InChI Key: |
ZOSQFDVXNQFKBY-CGAXJHMRSA-L |
|---|
| InChI: |
InChI=1S/C16H26N2O15P2/c1-6-4-18(16(24)17-14(6)23)10-3-8(19)9(31-10)5-29-34(25,26)33-35(27,28)32-15-13(22)12(21)11(20)7(2)30-15/h4,7-13,15,19-22H,3,5H2,1-2H3,(H,25,26)(H,27,28)(H,17,23,24)/p-2/t7-,8-,9+,10+,11-,12+,13+,15+/m0/s1 |
|---|
| CAS
number: |
2147-59-3 |
|---|
| IUPAC Name: | thymidine 5'-[3-(6-deoxy-β-L-mannopyranosyl) diphosphate] |
|---|
|
Traditional IUPAC Name: |
dtdp-L-rhamnose |
|---|
| SMILES: | CC1(=CN(C(=O)NC(=O)1)C3(CC(O)C(COP(=O)([O-])OP(=O)([O-])OC2(OC(C)C(O)C(O)C(O)2))O3)) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as pyrimidine nucleotide sugars. These are pyrimidine nucleotides bound to a saccharide derivative through the terminal phosphate group. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Nucleosides, nucleotides, and analogues |
|---|
| Sub Class | Pyrimidine nucleotides |
|---|
|
Direct Parent |
Pyrimidine nucleotide sugars |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Pyrimidine nucleotide sugar
- Pyrimidine 2'-deoxyribonucleoside diphosphate
- Pentose phosphate
- Monosaccharide phosphate
- Organic pyrophosphate
- Pyrimidone
- Monoalkyl phosphate
- Phosphoric acid ester
- Hydropyrimidine
- Monosaccharide
- Pyrimidine
- Organic phosphoric acid derivative
- Alkyl phosphate
- Oxane
- Heteroaromatic compound
- Vinylogous amide
- Oxolane
- Urea
- Lactam
- Secondary alcohol
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Polyol
- Organonitrogen compound
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organooxygen compound
- Organic nitrogen compound
- Organic oxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Giraud MF, Leonard GA, Field RA, Berlind C, Naismith JH: RmlC, the third enzyme of dTDP-L-rhamnose pathway, is a new class of epimerase. Nat Struct Biol. 2000 May;7(5):398-402. [10802738 ]
- Dong C, Beis K, Giraud MF, Blankenfeldt W, Allard S, Major LL, Kerr ID, Whitfield C, Naismith JH: A structural perspective on the enzymes that convert dTDP-d-glucose into dTDP-l-rhamnose. Biochem Soc Trans. 2003 Jun;31(Pt 3):532-6. [12773151 ]
|
|---|
| Synthesis Reference: |
Shibaev, V. N.; Kusov, Yu. Yu.; Eliseeva, G. I.; Petrenko, V. A. Synthesis of thymidine diphosphate rhamnose analogs. Ref. Dokl. Soobshch. - Mendeleevsk. S'ezd Obshch. Prikl. Khim., 11th (1975), 6 111. CODEN: 37MOAO CAN 88:191313 AN 1978:191313 |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|