|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110480 |
|---|
|
Identification |
|---|
| Name: |
adenylo-succinate |
|---|
| Description: | The N6-(1,2-dicarboxyethyl) derivative of adenosine 5'-monophosphate. |
|---|
|
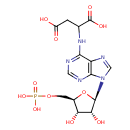
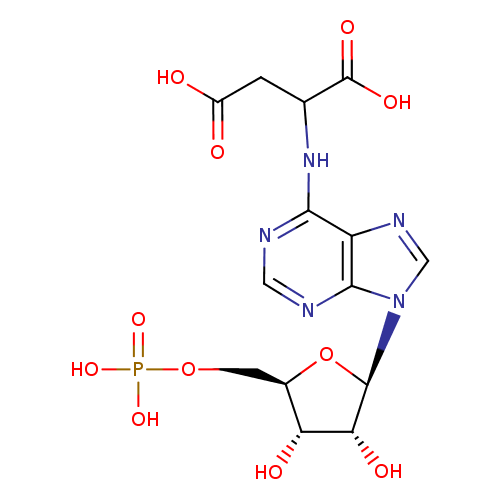
Structure |
|
|---|
| Synonyms: | -
N6-(1,2-dicarboxyethyl)-AMP
-
adenylo-succ
|
|---|
|
Chemical Formula: |
C14H14N5O11P
|
|---|
| Average Molecular Weight: |
459.26 |
|---|
| Monoisotopic Molecular
Weight: |
463.0740429569 |
|---|
| InChI Key: |
OFBHPPMPBOJXRT-DPXQIYNJSA-J |
|---|
| InChI: |
InChI=1S/C14H18N5O11P/c20-7(21)1-5(14(24)25)18-11-8-12(16-3-15-11)19(4-17-8)13-10(23)9(22)6(30-13)2-29-31(26,27)28/h3-6,9-10,13,22-23H,1-2H2,(H,20,21)(H,24,25)(H,15,16,18)(H2,26,27,28)/p-4/t5?,6-,9-,10-,13-/m1/s1 |
|---|
| CAS
number: |
19046-78-7 |
|---|
| IUPAC Name: | N- [9- [9- (5- (5- O- O- phosphono- phosphono- β- β- D- D- ribofuranosyl)- ribofuranosyl)- 9H- 9H- purin- purin- 6- 6- yl]aspartic acid yl]aspartic acid |
|---|
|
Traditional IUPAC Name: |
adenylosuccinate |
|---|
| SMILES: | C(OP(=O)([O-])[O-])C1(OC(C(O)C(O)1)N3(C=NC2(C(=NC=NC=23)NC(CC(=O)[O-])C(=O)[O-]))) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as purine ribonucleoside monophosphates. These are nucleotides consisting of a purine base linked to a ribose to which one monophosphate group is attached. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Nucleosides, nucleotides, and analogues |
|---|
| Sub Class | Purine nucleotides |
|---|
|
Direct Parent |
Purine ribonucleoside monophosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Purine ribonucleoside monophosphate
- Pentose phosphate
- Pentose-5-phosphate
- Aspartic acid or derivatives
- Glycosyl compound
- N-glycosyl compound
- 6-alkylaminopurine
- Monosaccharide phosphate
- Alpha-amino acid or derivatives
- 6-aminopurine
- Pentose monosaccharide
- Imidazopyrimidine
- Purine
- Aminopyrimidine
- Monoalkyl phosphate
- Secondary aliphatic/aromatic amine
- Pyrimidine
- N-substituted imidazole
- Dicarboxylic acid or derivatives
- Phosphoric acid ester
- Monosaccharide
- Organic phosphoric acid derivative
- Alkyl phosphate
- Imidolactam
- Heteroaromatic compound
- Azole
- Imidazole
- Oxolane
- Secondary alcohol
- 1,2-diol
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Carboxylic acid derivative
- Carboxylic acid
- Organic oxide
- Hydrocarbon derivative
- Carbonyl group
- Alcohol
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0ik9-0040900000-b82cfa15a82a71e3a806 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0udi-0190000000-9bcb8baa6f6c26df4465 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-03du-0920000000-f57e5dc2c827d4094209 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Nomura Y, Nozawa A, Tozawa Y (2014)Biochemical analyses of ppGpp effect on adenylosuccinate synthetases, key enzymes in purine biosynthesis in rice. Bioscience, biotechnology, and biochemistry 78, Pubmed: 25036129
- Boitz JM, Strasser R, Yates PA, Jardim A, Ullman B (2013)Adenylosuccinate synthetase and adenylosuccinate lyase deficiencies trigger growth and infectivity deficits in Leishmania donovani. The Journal of biological chemistry 288, Pubmed: 23404497
|
|---|
| Synthesis Reference: |
Buck, Ildiko; Reese, Colin B. An unambiguous synthesis of adenylosuccinic acid and its constituent nucleoside. Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999) (1990), (11), 2937-42. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|