|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110457 |
|---|
|
Identification |
|---|
| Name: |
N2 |
|---|
| Description: | An elemental molecule consisting of two trivalently-bonded nitrogen atoms. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
nitrogen molecule
-
nitrogen
|
|---|
|
Chemical Formula: |
N2
|
|---|
| Average Molecular Weight: |
28.013 |
|---|
| Monoisotopic Molecular
Weight: |
28.0061480104 |
|---|
| InChI Key: |
IJGRMHOSHXDMSA-UHFFFAOYSA-N |
|---|
| InChI: |
InChI=1S/N2/c1-2 |
|---|
| CAS
number: |
7727-37-9 |
|---|
| IUPAC Name: | dinitrogen |
|---|
|
Traditional IUPAC Name: |
nitrogen |
|---|
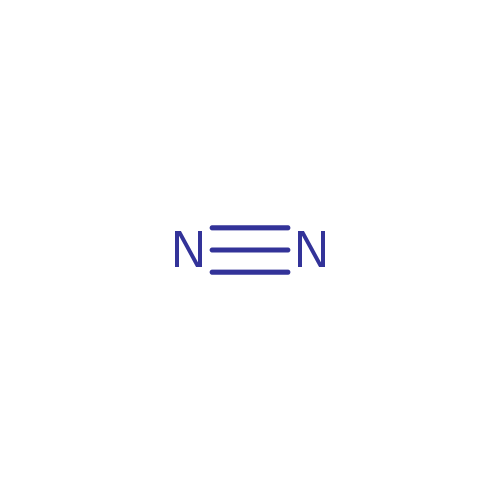
| SMILES: | N#N |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as other non-metal nitrides. These are inorganic compounds of nitrogen where nitrogen has a formal oxidation state of -3, and the heaviest atom bonded to it belongs to the class of 'other non-metals'. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Inorganic compounds |
|---|
|
Class |
Homogeneous non-metal compounds |
|---|
| Sub Class | Other non-metal organides |
|---|
|
Direct Parent |
Other non-metal nitrides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Other non-metal nitride
- Inorganic nitride
|
|---|
| Molecular Framework |
Not Available |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Liquid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
-210.01 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | -210.01 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 18.1 mg/mL at 21 °C | Not Available | | LogP | 0.67 | HANSCH,C ET AL. (1995) |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Pfreundt U, Stal LJ, Voß B, Hess WR (2012)Dinitrogen fixation in a unicellular chlorophyll d-containing cyanobacterium. The ISME journal 6, Pubmed: 22237545
- Paungfoo-Lonhienne C, Lonhienne TG, Rentsch D, Robinson N, Christie M, Webb RI, Gamage HK, Carroll BJ, Schenk PM, Schmidt S (2008)Plants can use protein as a nitrogen source without assistance from other organisms. Proceedings of the National Academy of Sciences of the United States of America 105, Pubmed: 18334638
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|