|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110431 |
|---|
|
Identification |
|---|
| Name: |
glutathione |
|---|
| Description: | A peptide anion obtained by deprotonation of both carboxy groups and protonation of the glutamyl amino group of glutathione; major species at pH 7.3. |
|---|
|
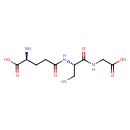
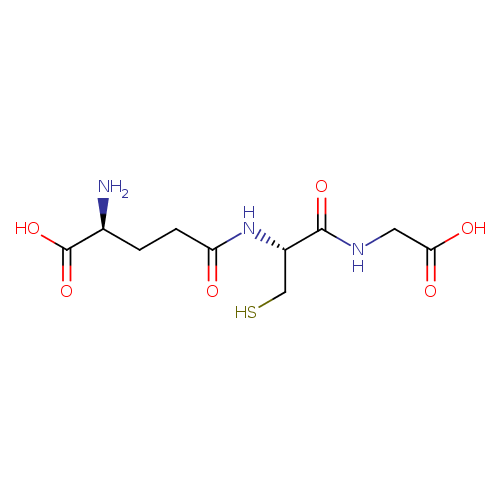
Structure |
|
|---|
| Synonyms: | -
γ-L-glutamyl-L-cysteinyl-glycine
-
GSH
-
reduced glutathione
-
glutathionate
|
|---|
|
Chemical Formula: |
C10H16N3O6S
|
|---|
| Average Molecular Weight: |
306.31 |
|---|
| Monoisotopic Molecular
Weight: |
308.091631016 |
|---|
| InChI Key: |
RWSXRVCMGQZWBV-WDSKDSINSA-M |
|---|
| InChI: |
InChI=1S/C10H17N3O6S/c11-5(10(18)19)1-2-7(14)13-6(4-20)9(17)12-3-8(15)16/h5-6,20H,1-4,11H2,(H,12,17)(H,13,14)(H,15,16)(H,18,19)/p-1/t5-,6-/m0/s1 |
|---|
| CAS
number: |
70-18-8 |
|---|
| IUPAC Name: | (2S)-2-amino-4-{[(1R)-1-[(carboxymethyl)carbamoyl]-2-sulfanylethyl]carbamoyl}butanoic acid |
|---|
|
Traditional IUPAC Name: |
glutathione |
|---|
| SMILES: | C(S)C(C(NCC([O-])=O)=O)NC(=O)CCC([N+])C([O-])=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as peptides. These are compounds containing an amide derived from two or more amino carboxylic acid molecules (the same or different) by formation of a covalent bond from the carbonyl carbon of one to the nitrogen atom of another. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Carboxylic acids and derivatives |
|---|
|
Direct Parent |
Peptides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Alpha peptide
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- Alpha-amino acid
- Alpha-amino acid or derivatives
- L-alpha-amino acid
- Dicarboxylic acid or derivatives
- Fatty acid
- Amino acid or derivatives
- Amino acid
- Alkylthiol
- Carboximidic acid
- Carboximidic acid derivative
- Carboxylic acid
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Hydrocarbon derivative
- Primary aliphatic amine
- Organic oxide
- Organic oxygen compound
- Organic nitrogen compound
- Carbonyl group
- Amine
- Organonitrogen compound
- Organooxygen compound
- Organosulfur compound
- Organopnictogen compound
- Primary amine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
195 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 195 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 292.5 mg/mL | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (x TMS) | splash10-0a4i-0900000000-5841845f736f9a667622 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) (x TMS) | splash10-0a4i-0900000000-bdecde153761cb67852e | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS | Not Available |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-004i-3795000000-d019cd7dcbad1f8a9e78 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-003r-9400000000-a83bf6292d41988256e3 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-001i-9000000000-305a92f8a9ffea58fa0e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-0a4i-0009000000-e950bfc5867b391c6960 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-004i-0910000000-83f6c079d1112e74ecf4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-003r-0910000000-5b243cf8bd357ab270b1 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-0a4i-0009000000-29ef335479f56b620d88 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-0a4i-0009001000-d1f5986166efa523d024 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-056s-0495300000-dba7be381fd1ef776527 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-004i-0920000000-de5b8a5a377324599b39 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Positive | splash10-053r-0007920000-7500cef211e48c8ea244 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-0a4i-0119003000-4eb7ed4e2a4cf6a83c66 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-0uki-0290000000-6893386899c6eed6a1a6 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-05g0-0190000000-cacc2de4ab18ed59798b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-0a4i-0009000000-6b4268add43ab66ef015 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-08fr-0015009000-a01bdc13a34d6ce8416f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-0uki-0290000000-ea94ec8247b4e025adbe | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-0a4i-0039210000-e1f721157a9ea89959d6 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT (LTQ Orbitrap XL, Thermo Scientfic) , Negative | splash10-0a59-0039210000-35ce450ea95922abf0e1 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-0a4i-0009000000-9b01fba547d1fcde113a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-0006-0952000000-4a2a42699cf4aab2c559 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-002f-2900000000-bd9ba27b48b1322b7618 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative | splash10-004m-5900000000-cc7184d5bba50e6e49d0 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Negative | splash10-0a4i-9200000000-aecd0eb18a10c3ffb7ab | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Uotila L (1973)Preparation and assay of glutathione thiol esters. Survey of human liver glutathione thiol esterases. Biochemistry 12, Pubmed: 4200890
- Uotila L (1973)Purification and characterization of S-2-hydroxyacylglutathione hydrolase (glyoxalase II) from human liver. Biochemistry 12, Pubmed: 4745654
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|