|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110423 |
|---|
|
Identification |
|---|
| Name: |
adenosylcobinamide-GDP |
|---|
| Description: | An organophosphate oxoanion that is the anion of adenosylcobinamide guanosyl diphosphate with overall charge −1, arising from deprotonation of the phosphate OH groups; major species at pH 7.3. |
|---|
|
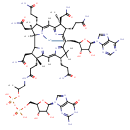
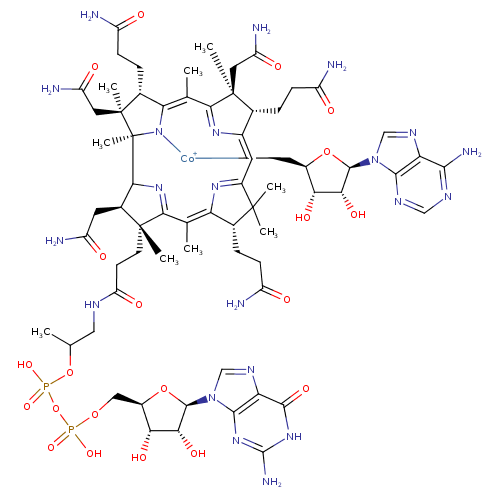
Structure |
|
|---|
| Synonyms: | |
|---|
|
Chemical Formula: |
C68H95N21O21P2Co
|
|---|
| Average Molecular Weight: |
1663.5 |
|---|
| Monoisotopic Molecular
Weight: |
1664.597512507 |
|---|
| InChI Key: |
IQTYKHRKNGVJEO-RRMAJTJESA-K |
|---|
| InChI: |
InChI=1S/C58H86N16O18P2.C10H12N5O3.Co/c1-25(91-94(87,88)92-93(85,86)89-23-33-45(82)46(83)52(90-33)74-24-67-44-50(74)71-53(65)72-51(44)84)22-66-41(81)16-17-55(6)31(18-38(62)78)49-58(9)57(8,21-40(64)80)30(12-15-37(61)77)43(73-58)27(3)48-56(7,20-39(63)79)28(10-13-35(59)75)32(68-48)19-34-54(4,5)29(11-14-36(60)76)42(69-34)26(2)47(55)70-49;1-4-6(16)7(17)10(18-4)15-3-14-5-8(11)12-2-13-9(5)15;/h19,24-25,28-31,33,45-46,49,52,82-83H,10-18,20-23H2,1-9H3,(H19,59,60,61,62,63,64,65,66,68,69,70,71,72,73,75,76,77,78,79,80,81,84,85,86,87,88);2-4,6-7,10,16-17H,1H2,(H2,11,12,13);/q;;+2/p-3/t25-,28-,29-,30-,31+,33-,45-,46-,49-,52-,55-,56+,57+,58+;4-,6-,7-,10-;/m11./s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | [(1R,3R,4R,6Z,8S,11Z,13S,14S,16Z,18S,19S)-4-(2-{[2-({[({[(2R,3S,4R,5R)-5-(2-amino-6-oxo-6,9-dihydro-1H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)propyl]carbamoyl}ethyl)-8,13,18-tris(2-carbamoylethyl)-3,14,19-tris(carbamoylmethyl)-1,4,6,9,9,14,16,19-octamethyl-20,21,22,23-tetraazapentacyclo[15.2.1.1?,??1?????1??,???tricosa-5(23),6,10(22),11,15(21),16-hexaen-20-yl]({[(2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl})cobaltylium |
|---|
|
Traditional IUPAC Name: |
[(1R,3R,4R,6Z,8S,11Z,13S,14S,16Z,18S,19S)-4-[2-({2-[({[(2R,3S,4R,5R)-5-(2-amino-6-oxo-1H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy(hydroxy)phosphoryl}oxy(hydroxy)phosphoryl)oxy]propyl}carbamoyl)ethyl]-8,13,18-tris(2-carbamoylethyl)-3,14,19-tris(carbamoylmethyl)-1,4,6,9,9,14,16,19-octamethyl-20,21,22,23-tetraazapentacyclo[15.2.1.1?,??1?????1??,???tricosa-5(23),6,10(22),11,15(21),16-hexaen-20-yl]({[(2S,3S,4R,5R)-5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl})cobaltylium |
|---|
| SMILES: | CC(OP([O-])(=O)OP([O-])(=O)OCC3(C(O)C(O)C(N2(C1(=C(C(=O)NC(N)=N1)N=C2)))O3))CNC(=O)CCC%11(C)(C(CC(=O)N)C%13(C%14(C)(C(C)(CC(N)=O)C(CCC(N)=O)C7(=[N+]([Co--]8%12(CC6(C(O)C(C(N5(C=NC4(C(N)=NC=NC=45)))O6)O))([N+]%10(C(=CC9(C(CCC(N)=O)C(C)(CC(N)=O)C(=C(C)7)[N+]8=9))C(C)(C)C(CCC(N)=O)C=%10C(C)=C%11N%12%13)))%14)))) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as purine ribonucleoside diphosphates. These are purine ribobucleotides with diphosphate group linked to the ribose moiety. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Nucleosides, nucleotides, and analogues |
|---|
| Sub Class | Purine nucleotides |
|---|
|
Direct Parent |
Purine ribonucleoside diphosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Purine ribonucleoside diphosphate
- Metallotetrapyrrole skeleton
- Purine ribonucleoside monophosphate
- Tetrapyrrole skeleton
- Pentose-5-phosphate
- Pentose phosphate
- 5'-deoxyribonucleoside
- Glycosyl compound
- N-glycosyl compound
- 6-aminopurine
- 6-oxopurine
- Monosaccharide phosphate
- Organic pyrophosphate
- Hypoxanthine
- Purine
- Imidazopyrimidine
- Phosphoethanolamine
- Aminopyrimidine
- Pyrimidone
- Monoalkyl phosphate
- Fatty amide
- Imidolactam
- Primary aromatic amine
- Monosaccharide
- N-substituted imidazole
- Organic phosphoric acid derivative
- Pyrimidine
- Alkyl phosphate
- Fatty acyl
- Phosphoric acid ester
- Pyrrolidine
- Pyrroline
- Heteroaromatic compound
- Imidazole
- Tetrahydrofuran
- Vinylogous amide
- Azole
- Lactam
- Amino acid or derivatives
- Carboxamide group
- Ketimine
- Secondary carboxylic acid amide
- Secondary alcohol
- Primary carboxylic acid amide
- Oxacycle
- Carboxylic acid derivative
- Organoheterocyclic compound
- Azacycle
- Organic metal salt
- Organic transition metal salt
- Organic nitrogen compound
- Organic oxygen compound
- Organic transition metal moeity
- Imine
- Organometallic compound
- Organonitrogen compound
- Organooxygen compound
- Primary amine
- Organic salt
- Organic cobalt salt
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Transition metal alkyl
- Organopnictogen compound
- Amine
- Alcohol
- Organic cation
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- adenosylcobalamin biosynthesis II (late cobalt incorporation)P381-PWY
- adenosylcobalamin salvage from cobinamide ICOBALSYN-PWY
- adenosylcobalamin biosynthesis from cobyrinate a,c-diamide IPWY-5509
- adenosylcobalamin biosynthesis from cobyrinate a,c-diamide IIPWY-5508
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|