|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110416 |
|---|
|
Identification |
|---|
| Name: |
cob(I)yrinate a,c-diamide |
|---|
| Description: | A precorrin carboxylic acid anion obtained by global deprotonation of the carboxy groups of cob(I)yrinate a,c diamide. It is the major microspecies at pH 7.3 (according to Marvin v 6.2.0.). |
|---|
|
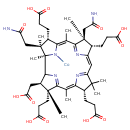
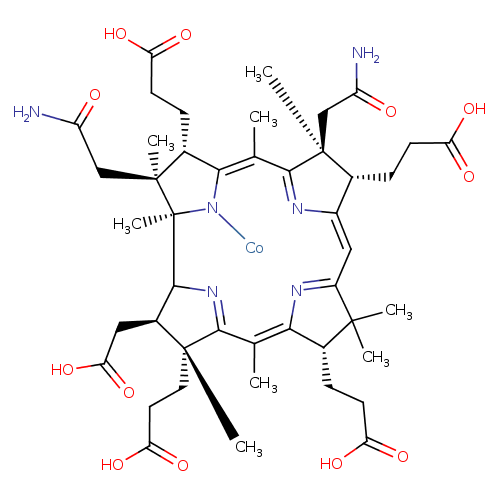
Structure |
|
|---|
| Synonyms: | -
cob(I)yrinic acid a,c-diamide
-
Cob(I)yrinate diamide
|
|---|
|
Chemical Formula: |
C45H56N6O12Co
|
|---|
| Average Molecular Weight: |
931.9 |
|---|
| Monoisotopic Molecular
Weight: |
936.3679466545 |
|---|
| InChI Key: |
NKLHEMWEQJCPPF-OKJGWHJPSA-H |
|---|
| InChI: |
InChI=1S/C45H62N6O12.Co/c1-21-36-24(10-13-32(56)57)41(3,4)28(49-36)18-27-23(9-12-31(54)55)43(6,19-29(46)52)39(48-27)22(2)37-25(11-14-33(58)59)44(7,20-30(47)53)45(8,51-37)40-26(17-35(62)63)42(5,38(21)50-40)16-15-34(60)61;/h18,23-26,40H,9-17,19-20H2,1-8H3,(H10,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63);/q;+1/p-6/t23-,24-,25-,26+,40-,42-,43+,44+,45+;/m1./s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | [(1R,3R,4R,8S,13S,14S,18S,19S)-14,19-bis(carbamoylmethyl)-4,8,13,18-tetrakis(2-carboxyethyl)-3-(carboxymethyl)-1,4,6,9,9,14,16,19-octamethyl-20,21,22,23-tetraazapentacyclo[15.2.1.1?,??1?????1??,???tricosa-5(23),6,10(22),11,15(21),16-hexaen-20-yl]cobalt |
|---|
|
Traditional IUPAC Name: |
[(1R,3R,4R,8S,13S,14S,18S,19S)-14,19-bis(carbamoylmethyl)-4,8,13,18-tetrakis(2-carboxyethyl)-3-(carboxymethyl)-1,4,6,9,9,14,16,19-octamethyl-20,21,22,23-tetraazapentacyclo[15.2.1.1?,??1?????1??,???tricosa-5(23),6,10(22),11,15(21),16-hexaen-20-yl]cobalt |
|---|
| SMILES: | CC6(=C7(C(C([CH]8(C2([N+]1([Co---]4([N+]3(C(=C(C)C=1C(CCC([O-])=O)C(CC(=O)N)(C)2)C(C(CCC(=O)[O-])C=3C=C5(C(C)(C)C(CCC(=O)[O-])C(=[N+]45)6))(CC(=O)N)C))N78))C))CC(=O)[O-])(C)CCC(=O)[O-])) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as precorrins. These are intermediates formed by methylation at one or more of the four rings prior to the formation of the macrocyclic corrin ring. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organoheterocyclic compounds |
|---|
| Sub Class | Tetrapyrroles and derivatives |
|---|
|
Direct Parent |
Precorrins |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Precorrin
- Metallotetrapyrrole skeleton
- Pentacarboxylic acid or derivatives
- Fatty amide
- Fatty acyl
- Pyrrolidine
- Pyrroline
- Carboxamide group
- Ketimine
- Primary carboxylic acid amide
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Azacycle
- Organic transition metal salt
- Organic metal salt
- Carboxylic acid derivative
- Carboxylic acid
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Carbonyl group
- Imine
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxide
- Organic salt
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -5 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- adenosylcobalamin biosynthesis II (late cobalt incorporation)P381-PWY
- adenosylcobalamin biosynthesis from cobyrinate a,c-diamide IPWY-5509
- adenosylcobalamin biosynthesis from cobyrinate a,c-diamide IIPWY-5508
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|