|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110383 |
|---|
|
Identification |
|---|
| Name: |
3'-dephospho-CoA |
|---|
| Description: | Dianion of 3'-dephospho-CoA. |
|---|
|
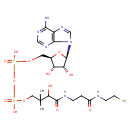
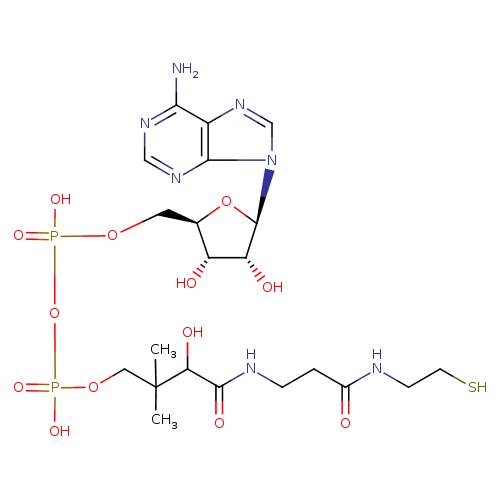
Structure |
|
|---|
| Synonyms: | -
3-dephospho-CoA
-
dephosphocoenzyme A
|
|---|
|
Chemical Formula: |
C21H33N7O13P2S
|
|---|
| Average Molecular Weight: |
685.54 |
|---|
| Monoisotopic Molecular
Weight: |
687.1488779572 |
|---|
| InChI Key: |
KDTSHFARGAKYJN-IBOSZNHHSA-L |
|---|
| InChI: |
InChI=1S/C21H35N7O13P2S/c1-21(2,16(32)19(33)24-4-3-12(29)23-5-6-44)8-39-43(36,37)41-42(34,35)38-7-11-14(30)15(31)20(40-11)28-10-27-13-17(22)25-9-26-18(13)28/h9-11,14-16,20,30-32,44H,3-8H2,1-2H3,(H,23,29)(H,24,33)(H,34,35)(H,36,37)(H2,22,25,26)/p-2/t11-,14-,15-,16+,20-/m1/s1 |
|---|
| CAS
number: |
3633-59-8 |
|---|
| IUPAC Name: | adenosine 5'- {3- {3- [(3R)- [(3R)- 3- 3- hydroxy- hydroxy- 2,2- 2,2- dimethyl- dimethyl- 4- 4- oxo- oxo- 4- 4- ({3- ({3- oxo- oxo- 3- 3- [(2- [(2- sulfanylethyl)amino]propyl}amino)butyl] diphosphate} sulfanylethyl)amino]propyl}amino)butyl] diphosphate} |
|---|
|
Traditional IUPAC Name: |
{[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy(hydroxy)phosphoryl}oxy(3-hydroxy-2,2-dimethyl-3-({2-[(2-sulfanylethyl)carbamoyl]ethyl}carbamoyl)propoxy)phosphinic acid |
|---|
| SMILES: | CC(C)(COP(=O)([O-])OP(=O)([O-])OCC1(OC(C(O)C(O)1)N3(C=NC2(C(N)=NC=NC=23))))C(O)C(=O)NCCC(=O)NCCS |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as purine ribonucleoside diphosphates. These are purine ribobucleotides with diphosphate group linked to the ribose moiety. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Nucleosides, nucleotides, and analogues |
|---|
| Sub Class | Purine nucleotides |
|---|
|
Direct Parent |
Purine ribonucleoside diphosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Purine ribonucleoside diphosphate
- Purine ribonucleoside monophosphate
- Pentose phosphate
- Pentose-5-phosphate
- Beta amino acid or derivatives
- Glycosyl compound
- N-glycosyl compound
- 6-aminopurine
- Monosaccharide phosphate
- Organic pyrophosphate
- Pentose monosaccharide
- Imidazopyrimidine
- Purine
- Aminopyrimidine
- Monoalkyl phosphate
- Fatty amide
- Monosaccharide
- N-acyl-amine
- N-substituted imidazole
- Organic phosphoric acid derivative
- Fatty acyl
- Imidolactam
- Phosphoric acid ester
- Primary aromatic amine
- Alkyl phosphate
- Pyrimidine
- Azole
- Oxolane
- Imidazole
- Heteroaromatic compound
- Carboxamide group
- Secondary alcohol
- Secondary carboxylic acid amide
- Amino acid or derivatives
- Azacycle
- Organoheterocyclic compound
- Carboxylic acid derivative
- Alkylthiol
- Oxacycle
- Organic nitrogen compound
- Organonitrogen compound
- Amine
- Organic oxygen compound
- Organooxygen compound
- Organosulfur compound
- Primary amine
- Organic oxide
- Hydrocarbon derivative
- Carbonyl group
- Alcohol
- Organopnictogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- Pantothenate and CoA Biosynthesis pae00770
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Haugen HF, Skrede S: Nucleotide pyrophosphatase and phosphodiesterase I. Demonstration of activity in normal serum, and an increase in cholestatic liver disease. Scand J Gastroenterol. 1976;11(2):121-7. [4880 ]
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|