|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110360 |
|---|
|
Identification |
|---|
| Name: |
pyochelin |
|---|
| Description: | A member of the class of thiazolidines that is (4R)-3-methyl-1,3-thiazolidine-4-carboxylic acid which is substituted at position 2 by a (4R)-2-(2-hydroxyphenyl)-4,5-dihydro-1,3-thiazol-4-yl group. A siderophore, it is it is produced by Pseudomonas aeruginosa (via condensation of salicylic acid and two molecules of cysteine) as a mixture of two easily interconvertible diastereoisomers, pyochelin I (major) and pyochelin II (minor). The enantiomeric compounds, enant-pyochelin, are produced by Pseudomonas fluorescens. |
|---|
|
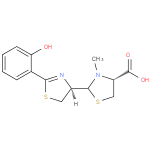
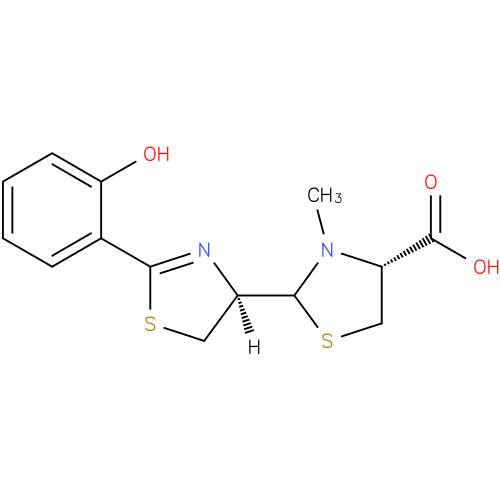
Structure |
|
|---|
| Synonyms: | - salicylic acid
- o-hydroxybenzoic acid
- 2-hydroxybenzoic acid
- SA
- 2-HBA
2-hydroxybenzoate- o-hydroxybenzoate'
- WIDTH
- 500);" onmouseout="return nd();">salicylate and two molecules of
L-cysteine. The structure of pyochelin was established as 2-(2-o-hydroxyphenyl-2-thiazolin-4-yl)-3-methylthiazolidine-4-carboxylic acid
[Cox81]. The compounds exists in two stereoisomers (
pyochelin and
enantio-pyochelin)
- which are produced by different organisms
[Youard07
-
Hoegy09].
|
|---|
|
Chemical Formula: |
C14H15N2O3S2
|
|---|
| Average Molecular Weight: |
324.0602337703 |
|---|
| Monoisotopic Molecular
Weight: |
324.0602337703 |
|---|
| InChI Key: |
NYBZAGXTZXPYND-GBIKHYSHSA-M |
|---|
| InChI: |
InChI=1S/C14H16N2O3S2/c1-16-10(14(18)19)7-21-13(16)9-6-20-12(15-9)8-4-2-3-5-11(8)17/h2-5,9-10,13,17H,6-7H2,1H3,(H,18,19)/p-1/t9-,10+,13-/m1/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | (4R)- 2- 2- [(4R)- [(4R)- 2- 2- (2- (2- hydroxyphenyl)- hydroxyphenyl)- 4,5- 4,5- dihydro- dihydro- 1,3- 1,3- thiazol- thiazol- 4- 4- yl]- yl]- 3- 3- methyl- methyl- 1,3- 1,3- thiazolidine- thiazolidine- 4- 4- carboxylic acid carboxylic acid |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | CN1(C(C([O-])=O)CS[CH]1[CH]3(CSC(C2(C(O)=CC=CC=2))=N3)) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as alpha amino acids and derivatives. These are amino acids in which the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (alpha carbon), or a derivative thereof. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
|
Class |
Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
|
Direct Parent |
Alpha amino acids and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Alpha-amino acid or derivatives
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Monocyclic benzene moiety
- Imidothiolactone
- Benzenoid
- Thiazolidine
- Meta-thiazoline
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Azacycle
- Organoheterocyclic compound
- Dialkylthioether
- Hemithioaminal
- Thioether
- Organic nitrogen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Organopnictogen compound
- Carbonyl group
- Organic anion
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
- a thiazoline siderophore (CPD-9992)
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Cox CD, Rinehart KL, Moore ML, Cook JC (1981)Pyochelin: novel structure of an iron-chelating growth promoter for Pseudomonas aeruginosa. Proceedings of the National Academy of Sciences of the United States of America 78, Pubmed: 6794030
- Schlegel K, Taraz K, Budzikiewicz H (2004)The stereoisomers of pyochelin, a siderophore of Pseudomonas aeruginosa. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine 17, Pubmed: 15259361
- Youard ZA, Wenner N, Reimmann C (2011)Iron acquisition with the natural siderophore enantiomers pyochelin and enantio-pyochelin in Pseudomonas species. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine 24, Pubmed: 21188474
- Hoegy F, Lee X, Noel S, Rognan D, Mislin GL, Reimmann C, Schalk IJ (2009)Stereospecificity of the siderophore pyochelin outer membrane transporters in fluorescent pseudomonads. The Journal of biological chemistry 284, Pubmed: 19297329
- Crosa JH, Walsh CT (2002)Genetics and assembly line enzymology of siderophore biosynthesis in bacteria. Microbiology and molecular biology reviews : MMBR 66, Pubmed: 12040125
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|