|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110359 |
|---|
|
Identification |
|---|
| Name: |
soyasapogenol E |
|---|
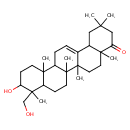
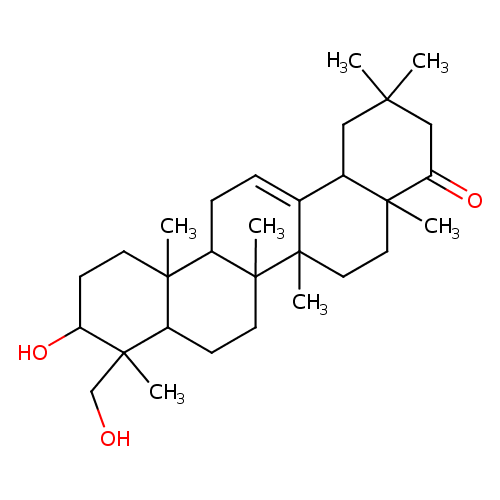
| Description: | A pentacyclic triterpenoid that is oleanane containing a double bond between positions 12 and 13, and is substituted by hydroxy groups at the 3β and 24-positions, and by an oxo group at position 22. |
|---|
|
Structure |
|
|---|
| Synonyms: | - 3,23-Dihydroxy-(3beta,4beta)-olean-12-en-22-one
- 3,24-Dihydroxy-12-oleanen-22-one
|
|---|
|
Chemical Formula: |
C30H48O3
|
|---|
| Average Molecular Weight: |
456.71 |
|---|
| Monoisotopic Molecular
Weight: |
456.3603454071 |
|---|
| InChI Key: |
FNRBOAGVUNHDIL-DYITYTDMSA-N |
|---|
| InChI: |
InChI=1S/C30H48O3/c1-25(2)16-20-19-8-9-22-27(4)12-11-23(32)28(5,18-31)21(27)10-13-30(22,7)29(19,6)15-14-26(20,3)24(33)17-25/h8,20-23,31-32H,9-18H2,1-7H3/t20?,21?,22?,23?,26-,27+,28-,29-,30-/m1/s1 |
|---|
| CAS
number: |
6750-59-0 |
|---|
| IUPAC Name: | (3β)-3,24-dihydroxyolean-12-en-22-one |
|---|
|
Traditional IUPAC Name: |
10-hydroxy-9-(hydroxymethyl)-2,2,4a,6a,6b,9,12a-heptamethyl-3,5,6,7,8,8a,10,11,12,12b,13,14b-dodecahydro-1H-picen-4-one |
|---|
| SMILES: | CC5(CC4(C3(=CCC2(C1(CCC(C(C1CCC2(C3(CCC4(C(C5)=O)C)C)C)(C)CO)O)C))))C |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as oleanane triterpenoids. These are triterpenoids with a structure based on the oleanane skeleton, an 4,4,6a,8a,11,14b-heptamethyl-hexadecahydropicene derivative. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
|
Class |
Prenol lipids |
|---|
| Sub Class | Triterpenoids |
|---|
|
Direct Parent |
Oleanane triterpenoids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Oleanane triterpenoid
- Steroid
- Cyclohexanone
- Cyclic alcohol
- Secondary alcohol
- Ketone
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework |
Aliphatic homopolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Kurosawa Y, Takahara H, Shiraiwa M (2002)UDP-glucuronic acid:soyasapogenol glucuronosyltransferase involved in saponin biosynthesis in germinating soybean seeds. Planta 215, Pubmed: 12172845
- Kinjo J, Yokomizo K, Hirakawa T, Shii Y, Nohara T, Uyeda M (2000)Anti-herpes virus activity of fabaceous triterpenoidal saponins'). Biological & pharmaceutical bulletin 23, Pubmed: 10919372
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|