|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110355 |
|---|
|
Identification |
|---|
| Name: |
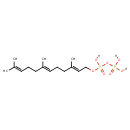
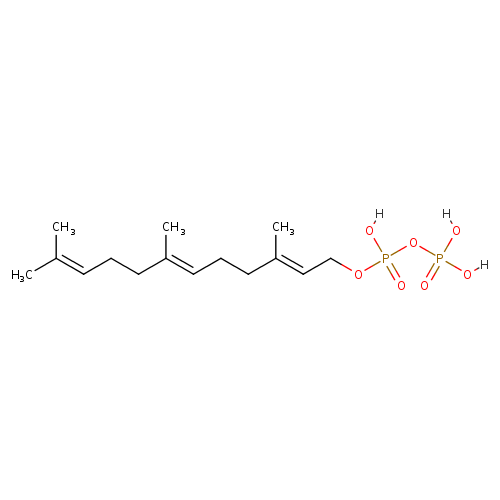
(2E,6E)-farnesyl diphosphate |
|---|
| Description: | An organophosphate oxoanion that is the trianion obtained by removal of the three protons from the diphosphate group of 2-trans,6-trans-farnesyl diphosphate. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
2-trans,6-trans-farnesyl diphosphate
-
2-trans,6-trans-farnesyl diphosphate
-
FPP
-
trans
- trans-farnesyl diphosphate
-
farnesyl-PP
-
farnesyl pyrophosphate
-
ω,E,E-farnesyl diphosphate
-
farnesyl diphosphate
-
(E,E)-farnesyl diphosphate
-
all-trans-farnesyl diphosphate
|
|---|
|
Chemical Formula: |
C15H25O7P2
|
|---|
| Average Molecular Weight: |
379.31 |
|---|
| Monoisotopic Molecular
Weight: |
382.1310262735 |
|---|
| InChI Key: |
VWFJDQUYCIWHTN-YFVJMOTDSA-K |
|---|
| InChI: |
InChI=1S/C15H28O7P2/c1-13(2)7-5-8-14(3)9-6-10-15(4)11-12-21-24(19,20)22-23(16,17)18/h7,9,11H,5-6,8,10,12H2,1-4H3,(H,19,20)(H2,16,17,18)/p-3/b14-9+,15-11+ |
|---|
| CAS
number: |
13058-04-3 |
|---|
| IUPAC Name: | (2E,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yl diphosphate |
|---|
|
Traditional IUPAC Name: |
[hydroxy([(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yl]oxy)phosphoryl]oxyphosphonic acid |
|---|
| SMILES: | CC(=CCCC(=CCCC(=CCOP(OP([O-])(=O)[O-])(=O)[O-])C)C)C |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as sesquiterpenoids. These are terpenes with three consecutive isoprene units. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Lipids and lipid-like molecules |
|---|
| Sub Class | Prenol lipids |
|---|
|
Direct Parent |
Sesquiterpenoids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Farsesane sesquiterpenoid
- Sesquiterpenoid
- Organic pyrophosphate
- Isoprenoid phosphate
- Monoalkyl phosphate
- Alkyl phosphate
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -3 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Notarnicola M, Messa C, Cavallini A, Bifulco M, Tecce MF, Eletto D, Di Leo A, Montemurro S, Laezza C, Caruso MG: Higher farnesyl diphosphate synthase activity in human colorectal cancer inhibition of cellular apoptosis. Oncology. 2004;67(5-6):351-8. [15713990 ]
- Shellman YG, Ribble D, Miller L, Gendall J, Vanbuskirk K, Kelly D, Norris DA, Dellavalle RP: Lovastatin-induced apoptosis in human melanoma cell lines. Melanoma Res. 2005 Apr;15(2):83-9. [15846140 ]
- Argmann CA, Edwards JY, Sawyez CG, O'Neil CH, Hegele RA, Pickering JG, Huff MW: Regulation of macrophage cholesterol efflux through hydroxymethylglutaryl-CoA reductase inhibition: a role for RhoA in ABCA1-mediated cholesterol efflux. J Biol Chem. 2005 Jun 10;280(23):22212-21. Epub 2005 Apr 6. [15817453 ]
- Reigard SA, Zahn TJ, Haworth KB, Hicks KA, Fierke CA, Gibbs RA: Interplay of isoprenoid and peptide substrate specificity in protein farnesyltransferase. Biochemistry. 2005 Aug 23;44(33):11214-23. [16101305 ]
- Tacer KF, Haugen TB, Baltsen M, Debeljak N, Rozman D: Tissue-specific transcriptional regulation of the cholesterol biosynthetic pathway leads to accumulation of testis meiosis-activating sterol (T-MAS). J Lipid Res. 2002 Jan;43(1):82-9. [11792726 ]
- Saisho Y, Morimoto A, Umeda T: Determination of farnesyl pyrophosphate in dog and human plasma by high-performance liquid chromatography with fluorescence detection. Anal Biochem. 1997 Oct 1;252(1):89-95. [9324945 ]
- Sanders JM, Song Y, Chan JM, Zhang Y, Jennings S, Kosztowski T, Odeh S, Flessner R, Schwerdtfeger C, Kotsikorou E, Meints GA, Gomez AO, Gonzalez-Pacanowska D, Raker AM, Wang H, van Beek ER, Papapoulos SE, Morita CT, Oldfield E: Pyridinium-1-yl bisphosphonates are potent inhibitors of farnesyl diphosphate synthase and bone resorption. J Med Chem. 2005 Apr 21;48(8):2957-63. [15828834 ]
- Fukuchi J, Song C, Ko AL, Liao S: Transcriptional regulation of farnesyl pyrophosphate synthase by liver X receptors. Steroids. 2003 Sep;68(7-8):685-91. [12957674 ]
|
|---|
| Synthesis Reference: |
Castillo-Bocanegra, Rafael. Synthesis and biological activity of farnesyl pyrophosphate analogs. (1977), 193 pp. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|