|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110253 |
|---|
|
Identification |
|---|
| Name: |
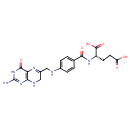
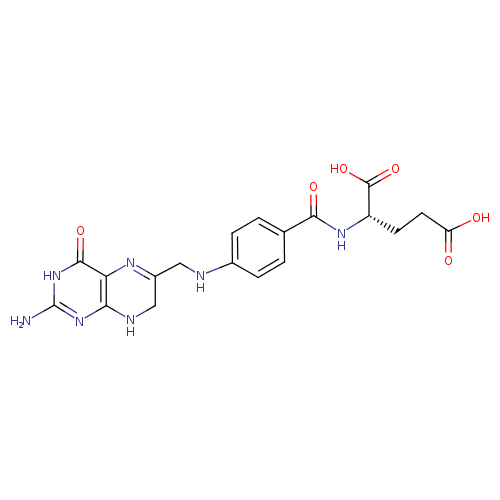
7,8-dihydrofolate monoglutamate |
|---|
| Description: | Dianion of dihydrofolic acid arising from deprotonation of both carboxylic acid functions. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
7,8-pteroylglutamic acid
-
7,8-pteroylglutamate
-
7,8-dihydropteroyl monoglutamate
-
H2PteGlu
-
H2PteGlu1
-
dihydrofolate
-
7,8-dihydropteroylglutamate
-
7,8-dihydrofolate
|
|---|
|
Chemical Formula: |
C19H19N7O6
|
|---|
| Average Molecular Weight: |
441.4 |
|---|
| Monoisotopic Molecular
Weight: |
443.1553314431 |
|---|
| InChI Key: |
OZRNSSUDZOLUSN-LBPRGKRZSA-L |
|---|
| InChI: |
InChI=1S/C19H21N7O6/c20-19-25-15-14(17(30)26-19)23-11(8-22-15)7-21-10-3-1-9(2-4-10)16(29)24-12(18(31)32)5-6-13(27)28/h1-4,12,21H,5-8H2,(H,24,29)(H,27,28)(H,31,32)(H4,20,22,25,26,30)/p-2/t12-/m0/s1 |
|---|
| CAS
number: |
4033-27-6 |
|---|
| IUPAC Name: | N- (4- (4- {[(2- {[(2- amino- amino- 4- 4- oxo- oxo- 3,4,7,8- 3,4,7,8- tetrahydropteridin- tetrahydropteridin- 6- 6- yl)methyl]amino}benzoyl)- yl)methyl]amino}benzoyl)- L- L- glutamate glutamate |
|---|
|
Traditional IUPAC Name: |
dihydrofolic acid |
|---|
| SMILES: | C(NC1(C=CC(C(=O)NC(C(=O)[O-])CCC([O-])=O)=CC=1))C3(CNC2(=C(C(=O)NC(N)=N2)N=3)) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as glutamic acid and derivatives. These are compounds containing glutamic acid or a derivative thereof resulting from reaction of glutamic acid at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Carboxylic acids and derivatives |
|---|
|
Direct Parent |
Glutamic acid and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Glutamic acid or derivatives
- Hippuric acid or derivatives
- Hippuric acid
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- Pterin
- Aminobenzamide
- Aminobenzoic acid or derivatives
- Pteridine
- Benzamide
- Benzoic acid or derivatives
- Benzoyl
- Phenylalkylamine
- Aniline or substituted anilines
- Hydroxypyrimidine
- Secondary aliphatic/aromatic amine
- Dicarboxylic acid or derivatives
- Benzenoid
- Pyrimidine
- Monocyclic benzene moiety
- Heteroaromatic compound
- Amino acid
- Secondary carboxylic acid amide
- Ketimine
- Carboxamide group
- Carboxylic acid
- Propargyl-type 1,3-dipolar organic compound
- Secondary amine
- Organoheterocyclic compound
- Organic 1,3-dipolar compound
- Azacycle
- Organic oxygen compound
- Imine
- Hydrocarbon derivative
- Carbonyl group
- Amine
- Organic oxide
- Organonitrogen compound
- Organopnictogen compound
- Organooxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Navarro-Peran E, Cabezas-Herrera J, Garcia-Canovas F, Durrant MC, Thorneley RN, Rodriguez-Lopez JN: The antifolate activity of tea catechins. Cancer Res. 2005 Mar 15;65(6):2059-64. [15781612 ]
|
|---|
| Synthesis Reference: |
Smith, Karin; Scrimgeour, K. G.; Huennekens, F. M. Folic acid coenzymes and one-carbon metabolism. XV. Synthesis of a new form of dihydrofolate. Biochemical and Biophysical Research Communications (1963), 11(5), 388-92. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|