|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110249 |
|---|
|
Identification |
|---|
| Name: |
tetrahydropteroyl tri-L-glutamate |
|---|
| Description: | Tetracarboxylate anion of tetrahydropteroyltri-L-glutamic acid; major species at pH 7.3. |
|---|
|
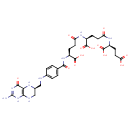
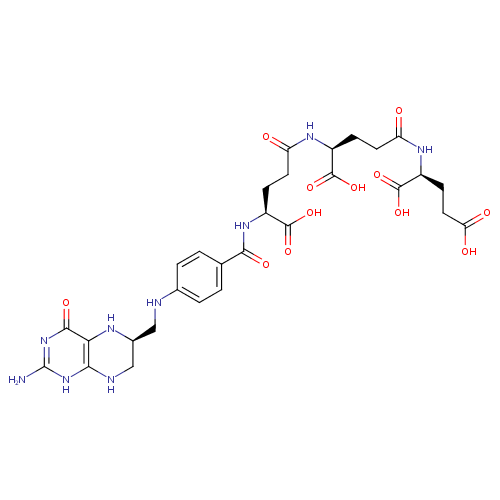
Structure |
|
|---|
| Synonyms: | |
|---|
|
Chemical Formula: |
C29H33N9O12
|
|---|
| Average Molecular Weight: |
699.63 |
|---|
| Monoisotopic Molecular
Weight: |
703.2561676997 |
|---|
| InChI Key: |
RXWVHRYZTWZATH-XSLAGTTESA-J |
|---|
| InChI: |
InChI=1S/C29H37N9O12/c30-29-37-23-22(25(44)38-29)33-15(12-32-23)11-31-14-3-1-13(2-4-14)24(43)36-18(28(49)50)6-9-20(40)34-16(26(45)46)5-8-19(39)35-17(27(47)48)7-10-21(41)42/h1-4,15-18,31,33H,5-12H2,(H,34,40)(H,35,39)(H,36,43)(H,41,42)(H,45,46)(H,47,48)(H,49,50)(H4,30,32,37,38,44)/p-4/t15-,16-,17-,18-/m0/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | (2S)-2-[(4S)-4-[(4S)-4-{[4-({[(6S)-2-amino-4-oxo-1,4,5,6,7,8-hexahydropteridin-6-yl]methyl}amino)phenyl]formamido}-4-carboxybutanamido]-4-carboxybutanamido]pentanedioic acid |
|---|
|
Traditional IUPAC Name: |
(2S)-2-[(4S)-4-[(4S)-4-{[4-({[(6S)-2-amino-4-oxo-5,6,7,8-tetrahydro-1H-pteridin-6-yl]methyl}amino)phenyl]formamido}-4-carboxybutanamido]-4-carboxybutanamido]pentanedioic acid |
|---|
| SMILES: | C([CH]2(NC1(C(NC(=NC=1NC2)N)=O)))NC3(=CC=C(C(NC(C(=O)[O-])CCC(NC(C(=O)[O-])CCC(NC(C(=O)[O-])CCC([O-])=O)=O)=O)=O)C=C3) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as tetrahydrofolic acids and derivatives. These are heterocyclic compounds based on the 5,6,7,8-tetrahydropteroic acid skeleton conjugated with at least one L-glutamic acid unit (or a derivative thereof) . |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organoheterocyclic compounds |
|---|
| Sub Class | Pteridines and derivatives |
|---|
|
Direct Parent |
Tetrahydrofolic acids and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Tetrahydrofolic acid or derivatives
- Alpha-oligopeptide
- Gamma-glutamyl alpha-amino acid
- Glutamic acid or derivatives
- Glutamine or derivatives
- N-acyl-alpha amino acid or derivatives
- Tetracarboxylic acid or derivatives
- N-acyl-alpha-amino acid
- Hippuric acid
- Hippuric acid or derivatives
- N-acyl-l-alpha-amino acid
- Aminobenzamide
- Alpha-amino acid or derivatives
- Aminobenzoic acid or derivatives
- Benzoic acid or derivatives
- Benzamide
- Phenylalkylamine
- Aniline or substituted anilines
- Benzoyl
- Pyrimidone
- Secondary aliphatic/aromatic amine
- Aminopyrimidine
- Monocyclic benzene moiety
- Fatty amide
- Fatty acyl
- N-acyl-amine
- Benzenoid
- Primary aromatic amine
- Pyrimidine
- Vinylogous amide
- Heteroaromatic compound
- Secondary carboxylic acid amide
- Amino acid or derivatives
- Amino acid
- Carboxamide group
- Secondary amine
- Azacycle
- Carboxylic acid
- Carboxylic acid derivative
- Organopnictogen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic nitrogen compound
- Amine
- Organic oxygen compound
- Organonitrogen compound
- Organooxygen compound
- Primary amine
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -4 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|