|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110217 |
|---|
|
Identification |
|---|
| Name: |
lipoamide |
|---|
| Description: | A monocarboxylic acid amide resulting from the formal condensation of the carboxy group of lipoic acid with ammonia. |
|---|
|
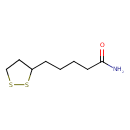
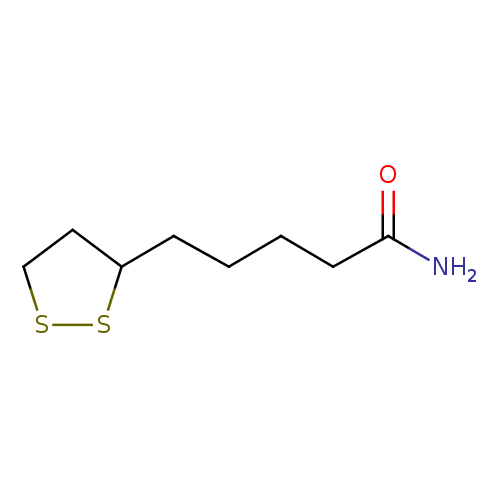
Structure |
|
|---|
| Synonyms: | -
5-(1,2-dithiolan-3-yl)-pentanamide
-
DL-lipoamide
-
6,8-dithiooctanoic amide
|
|---|
|
Chemical Formula: |
C8H15NOS2
|
|---|
| Average Molecular Weight: |
205.33 |
|---|
| Monoisotopic Molecular
Weight: |
205.0595054888 |
|---|
| InChI Key: |
FCCDDURTIIUXBY-SSDOTTSWSA-N |
|---|
| InChI: |
InChI=1S/C8H15NOS2/c9-8(10)4-2-1-3-7-5-6-11-12-7/h7H,1-6H2,(H2,9,10)/t7-/m1/s1 |
|---|
| CAS
number: |
940-69-2 |
|---|
| IUPAC Name: | 5-(1,2-dithiolan-3-yl)pentanamide |
|---|
|
Traditional IUPAC Name: |
lipoamide |
|---|
| SMILES: | C1(CC(CCCCC(N)=O)SS1) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as lipoamides. These are compounds containing a lipoamide moiety, which consists of a pentanamide attached to the C3 carbon atom of a 1,2-dithiolane ring. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organoheterocyclic compounds |
|---|
| Sub Class | Dithiolanes |
|---|
|
Direct Parent |
Lipoamides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Lipoamide
- Fatty amide
- Fatty acyl
- 1,2-dithiolane
- Carboxamide group
- Organic disulfide
- Primary carboxylic acid amide
- Carboxylic acid derivative
- Carbonyl group
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
126.0 - 129.0 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 126.0 - 129.0 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (2 TMS) | splash10-014i-6963000000-7e1887d53c2801b09961 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (1 TMS) | splash10-017l-3920000000-a3a7a08acf06d41c9b73 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS | Not Available |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-000i-0900000000-24fca44fc025dbd6be18 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0ziu-9700000000-7dfd23f450bdfd581100 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-056u-9000000000-13583c4eb6b06a56c392 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Positive | splash10-052r-2920000000-a4fb7176abf63e581f5e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Positive | splash10-000i-2900000000-10a079a84f024494ae23 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Positive | splash10-0zfr-9800000000-b01332176d6829b1eaa1 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Positive | splash10-0udl-9400000000-7a03c741edae36dff9cf | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Positive | splash10-0a5c-9100000000-7b44dfbfcbb0cc516254 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-IT (LC/MSD Trap XCT, Agilent Technologies) , Positive | splash10-000i-0900000000-50512172ce038bce2995 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-IT (LC/MSD Trap XCT, Agilent Technologies) , Positive | splash10-03di-0900000000-7652a33c17a37b6914aa | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-IT (LC/MSD Trap XCT, Agilent Technologies) , Positive | splash10-0udi-0900000000-0df59a6540bede8a4a79 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | Not Available |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Przybyla-Zawislak B, Gadde DM, Ducharme K, McCammon MT (1999)Genetic and biochemical interactions involving tricarboxylic acid cycle (TCA) function using a collection of mutants defective in all TCA cycle genes. Genetics 152, Pubmed: 10224250
- Shen W, Hao J, Feng Z, Tian C, Chen W, Packer L, Shi X, Zang W, Liu J (2011)Lipoamide or lipoic acid stimulates mitochondrial biogenesis in 3T3-L1 adipocytes via the endothelial NO synthase-cGMP-protein kinase G signalling pathway. British journal of pharmacology 162, Pubmed: 21108628
- Feeney MA, Veeravalli K, Boyd D, Gon S, Faulkner MJ, Georgiou G, Beckwith J (2011)Repurposing lipoic acid changes electron flow in two important metabolic pathways of Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America 108, Pubmed: 21521794
|
|---|
| Synthesis Reference: |
Xu, Yaming; Li, Zhitian; Gu, Yunlong. Synthesis of thioctamide. Faming Zhuanli Shenqing Gongkai Shuomingshu (1997), 5 pp. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|