|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110212 |
|---|
|
Identification |
|---|
| Name: |
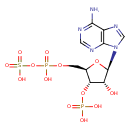
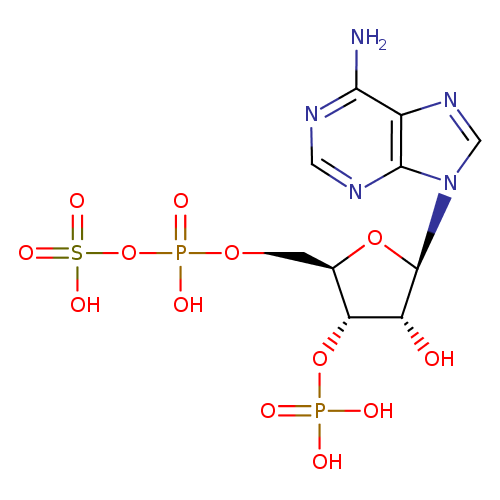
3'-phosphoadenylyl-sulfate |
|---|
| Description: | A quadruply-charged organophosphate oxoanion arising from deprotonation of the phosphate and sufate groups of 3'-phosphonato-5'-adenylyl sulfate; major species at pH 7.3. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
phosphoadenosine-5'-phosphosulfate
-
PAPS
-
phosphoadenosine phosphosulfate
-
3'-phosphoadenosine-5'-phosphosulfate
-
3'-phosphoadenylyl sulfate
-
3'-phospho-5'-adenylyl sulfate
|
|---|
|
Chemical Formula: |
C10H11N5O13P2S
|
|---|
| Average Molecular Weight: |
503.23 |
|---|
| Monoisotopic Molecular
Weight: |
506.9862293048 |
|---|
| InChI Key: |
GACDQMDRPRGCTN-KQYNXXCUSA-J |
|---|
| InChI: |
InChI=1S/C10H15N5O13P2S/c11-8-5-9(13-2-12-8)15(3-14-5)10-6(16)7(27-29(17,18)19)4(26-10)1-25-30(20,21)28-31(22,23)24/h2-4,6-7,10,16H,1H2,(H,20,21)(H2,11,12,13)(H2,17,18,19)(H,22,23,24)/p-4/t4-,6-,7-,10-/m1/s1 |
|---|
| CAS
number: |
482-67-7 |
|---|
| IUPAC Name: | 3'-O-phosphonato-5'-O-[(sulfonatooxy)phosphinato]adenosine |
|---|
|
Traditional IUPAC Name: |
3'-phosphoadenylyl sulfate |
|---|
| SMILES: | C(OP(=O)([O-])OS(=O)(=O)[O-])C1(C(OP(=O)([O-])[O-])C(O)C(O1)N3(C=NC2(C(N)=NC=NC=23))) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as purine ribonucleoside 3',5'-bisphosphates. These are purine ribobucleotides with one phosphate group attached to 3' and 5' hydroxyl groups of the ribose moiety. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Nucleosides, nucleotides, and analogues |
|---|
| Sub Class | Purine nucleotides |
|---|
|
Direct Parent |
Purine ribonucleoside 3',5'-bisphosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Purine ribonucleoside 3',5'-bisphosphate
- Purine ribonucleoside monophosphate
- Ribonucleoside 3'-phosphate
- Pentose phosphate
- Pentose-5-phosphate
- Glycosyl compound
- N-glycosyl compound
- 6-aminopurine
- Monosaccharide phosphate
- Pentose monosaccharide
- Imidazopyrimidine
- Purine
- Aminopyrimidine
- Monoalkyl phosphate
- Monosaccharide
- N-substituted imidazole
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Primary aromatic amine
- Imidolactam
- Alkyl phosphate
- Pyrimidine
- Oxolane
- Azole
- Organic sulfuric acid or derivatives
- Imidazole
- Heteroaromatic compound
- Secondary alcohol
- Azacycle
- Oxacycle
- Organoheterocyclic compound
- Organic oxide
- Organic nitrogen compound
- Hydrocarbon derivative
- Alcohol
- Amine
- Organic oxygen compound
- Primary amine
- Organonitrogen compound
- Organopnictogen compound
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -4 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Emmi L, Bergamini C, Spinelli A, Liotta F, Marchione T, Caldini A, Fanelli A, De Cristofaro MT, Dal Pozzo G: Possible pathogenetic role of activated platelets in the primary antiphospholipid syndrome involving the central nervous system. Ann N Y Acad Sci. 1997 Aug 14;823:188-200. [9292045 ]
- Fanelli A, Bergamini C, Rapi S, Caldini A, Spinelli A, Buggiani A, Emmi L: Flow cytometric detection of circulating activated platelets in primary antiphospholipid syndrome. Correlation with thrombocytopenia and anticardiolipin antibodies. Lupus. 1997;6(3):261-7. [9104734 ]
- Joseph JE, Harrison P, Mackie IJ, Isenberg DA, Machin SJ: Increased circulating platelet-leucocyte complexes and platelet activation in patients with antiphospholipid syndrome, systemic lupus erythematosus and rheumatoid arthritis. Br J Haematol. 2001 Nov;115(2):451-9. [11703349 ]
- Suarez IM, Diaz RA, Aguayo Canela D, Pujol de la Llave E: Correction of severe thrombocytopenia with chloroquine in the primary antiphospholipid syndrome. Lupus. 1996 Feb;5(1):81-3. [8646233 ]
- Khoo BY, Sit KH, Wong KP: Does PAPS generation determine the overall sulfate conjugation in human platelets? Life Sci. 1988;42(23):2389-95. [3131608 ]
- Wong KP, Khoo BY, Sit KH: Biosynthesis of PAPS in vitro by human liver. Measurement by two independent assay procedures. Biochem Pharmacol. 1991 Jan 1;41(1):63-9. [1846073 ]
- Cappiello M, Franchi M, Rane A, Pacifici GM: Sulphotransferase and its substrate: adenosine-3'-phosphate-5'-phosphosulphate in human fetal liver and placenta. Dev Pharmacol Ther. 1990;14(1):62-5. [2311482 ]
- Cappiello M, Franchi M, Giuliani L, Pacifici GM: Distribution of 2-naphthol sulphotransferase and its endogenous substrate adenosine 3'-phosphate 5'-phosphosulphate in human tissues. Eur J Clin Pharmacol. 1989;37(3):317-20. [2612547 ]
- Carlier M, Squifflet JP, Pirson Y, Gribomont B, Alexandre GP: Maximal hydration during anesthesia increases pulmonary arterial pressures and improves early function of human renal transplants. Transplantation. 1982 Oct;34(4):201-4. [6755828 ]
|
|---|
| Synthesis Reference: |
Lin, Chun-Hung; Shen, Gwo-Jenn; Garcia-Junceda, Eduardo; Wong, Chi-Huey. Enzymic Synthesis and Regeneration of 3'-Phosphoadenosine 5'-Phosphosulfate (PAPS) for Regioselective Sulfation of Oligosaccharides. Journal of the American Chemical Society (1995), |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|