|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110196 |
|---|
|
Identification |
|---|
| Name: |
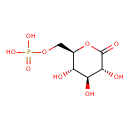
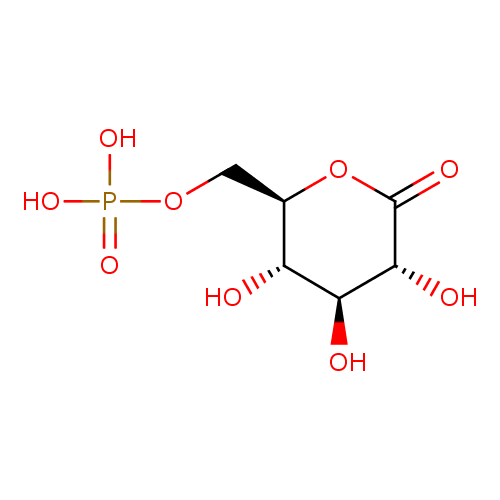
6-phospho D-glucono-1,5-lactone |
|---|
| Description: | Dianion of 6-O-phosphono-D-glucono-1,5-lactone arising from deprotonation of the phosphate OH groups; major species at pH 7.3. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
6-phosphogluconolactone
-
D-6-phosphoglucono-δ-lactone
-
D-6-P-glucono-δ-lactone
-
D-glucono-1,5-lactone 6-phosphate
-
D-glucono-δ-lactone 6-phosphate
-
D-6-phospho-glucono-δ-lactone
|
|---|
|
Chemical Formula: |
C6H9O9P
|
|---|
| Average Molecular Weight: |
256.11 |
|---|
| Monoisotopic Molecular
Weight: |
258.014068462 |
|---|
| InChI Key: |
IJOJIVNDFQSGAB-SQOUGZDYSA-L |
|---|
| InChI: |
InChI=1S/C6H11O9P/c7-3-2(1-14-16(11,12)13)15-6(10)5(9)4(3)8/h2-5,7-9H,1H2,(H2,11,12,13)/p-2/t2-,3-,4+,5-/m1/s1 |
|---|
| CAS
number: |
2641-81-8 |
|---|
| IUPAC Name: | [(2R,3S,4S,5R)-3,4,5-trihydroxy-6-oxooxan-2-yl]methyl phosphate |
|---|
|
Traditional IUPAC Name: |
6-phosphogluconolactone |
|---|
| SMILES: | C(OP([O-])(=O)[O-])C1(C(O)C(O)C(O)C(=O)O1) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as hexose phosphates. These are carbohydrate derivatives containing a hexose substituted by one or more phosphate groups. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic oxygen compounds |
|---|
| Sub Class | Organooxygen compounds |
|---|
|
Direct Parent |
Hexose phosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Hexose phosphate
- Gluconolactone
- Monosaccharide phosphate
- Delta valerolactone
- Delta_valerolactone
- Monoalkyl phosphate
- Alkyl phosphate
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Oxane
- Secondary alcohol
- Carboxylic acid ester
- Lactone
- Organoheterocyclic compound
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Oxacycle
- Polyol
- Hydrocarbon derivative
- Organic oxide
- Alcohol
- Carbonyl group
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Nakayama Y, Kinoshita A, Tomita M: Dynamic simulation of red blood cell metabolism and its application to the analysis of a pathological condition. Theor Biol Med Model. 2005 May 9;2(1):18. [15882454 ]
- Beutler E, Kuhl W: Characteristics and significance of the reverse glucose-6-phosphate dehydrogenase reaction. J Lab Clin Med. 1986 Jun;107(6):502-7. [3711719 ]
- Rakitzis ET, Papandreou P: Kinetic analysis of 6-phosphogluconolactone hydrolysis in hemolysates. Biochem Mol Biol Int. 1995 Nov;37(4):747-55. [8589648 ]
- Miclet E, Stoven V, Michels PA, Opperdoes FR, Lallemand JY, Duffieux F: NMR spectroscopic analysis of the first two steps of the pentose-phosphate pathway elucidates the role of 6-phosphogluconolactonase. J Biol Chem. 2001 Sep 14;276(37):34840-6. Epub 2001 Jul 16. [11457850 ]
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|