|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110176 |
|---|
|
Identification |
|---|
| Name: |
3-ureidopropanoate |
|---|
| Description: | A monocarboxylic acid anion that is the conjugate base of N-carbamoyl-β-alanine arising from deprotonation of the carboxy group. |
|---|
|
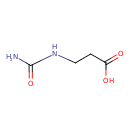
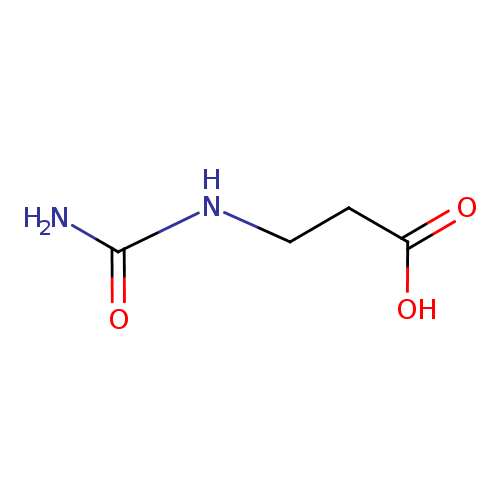
Structure |
|
|---|
| Synonyms: | -
3-ureidopropionate
-
β-ureidopropionic acid
-
N-carbamoyl-β-alanine
|
|---|
|
Chemical Formula: |
C4H7N2O3
|
|---|
| Average Molecular Weight: |
131.11 |
|---|
| Monoisotopic Molecular
Weight: |
132.0534921335 |
|---|
| InChI Key: |
JSJWCHRYRHKBBW-UHFFFAOYSA-M |
|---|
| InChI: |
InChI=1S/C4H8N2O3/c5-4(9)6-2-1-3(7)8/h1-2H2,(H,7,8)(H3,5,6,9)/p-1 |
|---|
| CAS
number: |
462-88-4 |
|---|
| IUPAC Name: | 3-(carbamoylamino)propanoate |
|---|
|
Traditional IUPAC Name: |
ureidopropionic acid |
|---|
| SMILES: | C(NC(=O)N)CC([O-])=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as ureas. These are compounds containing two amine groups joined by a carbonyl (C=O) functional group. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Organic carbonic acids and derivatives |
|---|
|
Direct Parent |
Ureas |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Urea
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
170 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 170 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 20.9 mg/mL | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Moolenaar SH, Gohlich-Ratmann G, Engelke UF, Spraul M, Humpfer E, Dvortsak P, Voit T, Hoffmann GF, Brautigam C, van Kuilenburg AB, van Gennip A, Vreken P, Wevers RA: beta-Ureidopropionase deficiency: a novel inborn error of metabolism discovered using NMR spectroscopy on urine. Magn Reson Med. 2001 Nov;46(5):1014-7. [11675655 ]
- Sparidans RW, Bosch TM, Jorger M, Schellens JH, Beijnen JH: Liquid chromatography-tandem mass spectrometric assay for the analysis of uracil, 5,6-dihydrouracil and beta-ureidopropionic acid in urine for the measurement of the activities of the pyrimidine catabolic enzymes. J Chromatogr B Analyt Technol Biomed Life Sci. 2006 Jul 24;839(1-2):45-53. Epub 2006 Feb 28. [16513432 ]
- Ito S, Kawamura T, Inada M, Inoue Y, Hirao Y, Koga T, Kunizaki J, Shimizu T, Sato H: Physiologically based pharmacokinetic modelling of the three-step metabolism of pyrimidine using C-uracil as an in vivo probe. Br J Clin Pharmacol. 2005 Dec;60(6):584-93. [16305582 ]
- Hofmann U, Schwab M, Seefried S, Marx C, Zanger UM, Eichelbaum M, Murdter TE: Sensitive method for the quantification of urinary pyrimidine metabolites in healthy adults by gas chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2003 Jul 5;791(1-2):371-80. [12798197 ]
- Malet-Martino MC, Armand JP, Lopez A, Bernadou J, Beteille JP, Bon M, Martino R: Evidence for the importance of 5'-deoxy-5-fluorouridine catabolism in humans from 19F nuclear magnetic resonance spectrometry. Cancer Res. 1986 Apr;46(4 Pt 2):2105-12. [2936452 ]
- Desmoulin F, Gilard V, Malet-Martino M, Martino R: Metabolism of capecitabine, an oral fluorouracil prodrug: (19)F NMR studies in animal models and human urine. Drug Metab Dispos. 2002 Nov;30(11):1221-9. [12386128 ]
|
|---|
| Synthesis Reference: |
w-Ureido carboxylic acids. (1962), 3 pp. GB 913713 19621228 CAN 58:72975 AN 1963:72975 |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|