|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110173 |
|---|
|
Identification |
|---|
| Name: |
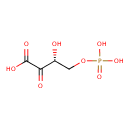
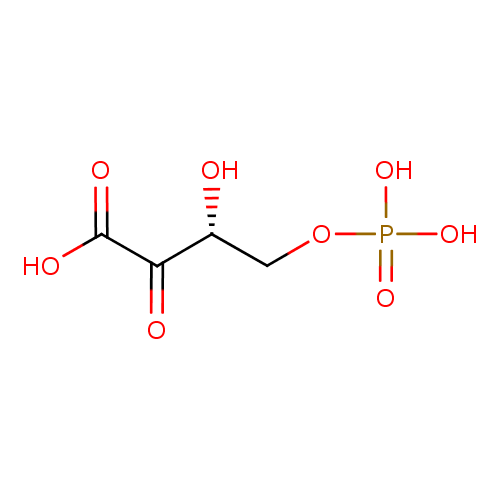
2-oxo-3-hydroxy-4-phosphobutanoate |
|---|
| Description: | A 2-oxo monocarboxylic acid that is 2-oxobutanoic acid which is substituted by a phosphonooxy group at position 4 and a hydroxy group at the 3-pro-R position. |
|---|
|
Structure |
|
|---|
| Synonyms: | -
3-hydroxy-4-phospho-hydroxy-α-ketobutyrate
-
(3R)-3-hydroxy-2-oxo-4 phosphonooxybutanoate
|
|---|
|
Chemical Formula: |
C4H4O8P
|
|---|
| Average Molecular Weight: |
211.04 |
|---|
| Monoisotopic Molecular
Weight: |
213.9878537115 |
|---|
| InChI Key: |
MZJFVXDTNBHTKZ-UWTATZPHSA-K |
|---|
| InChI: |
InChI=1S/C4H7O8P/c5-2(3(6)4(7)8)1-12-13(9,10)11/h2,5H,1H2,(H,7,8)(H2,9,10,11)/p-3/t2-/m1/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | (3R)-3-hydroxy-2-oxo-4-(phosphonooxy)butanoic acid |
|---|
|
Traditional IUPAC Name: |
(3R)-3-hydroxy-2-oxo-4-(phosphonooxy)butanoic acid |
|---|
| SMILES: | C(C(C(C([O-])=O)=O)O)OP([O-])(=O)[O-] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as short-chain keto acids and derivatives. These are keto acids with an alkyl chain the contains less than 6 carbon atoms. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Keto acids and derivatives |
|---|
|
Direct Parent |
Short-chain keto acids and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Monoalkyl phosphate
- Short-chain keto acid
- Beta-hydroxy acid
- Acyloin
- Alpha-keto acid
- Alkyl phosphate
- Phosphoric acid ester
- Hydroxy acid
- Organic phosphoric acid derivative
- Monosaccharide
- Alpha-hydroxy ketone
- Secondary alcohol
- Ketone
- Carboxylic acid
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Organooxygen compound
- Carbonyl group
- Organic oxygen compound
- Hydrocarbon derivative
- Organic oxide
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- pyridoxal 5'-phosphate biosynthesis IPYRIDOXSYN-PWY
- superpathway of pyridoxal 5'-phosphate biosynthesis and salvagePWY0-845
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|