|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110169 |
|---|
|
Identification |
|---|
| Name: |
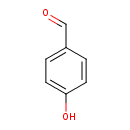
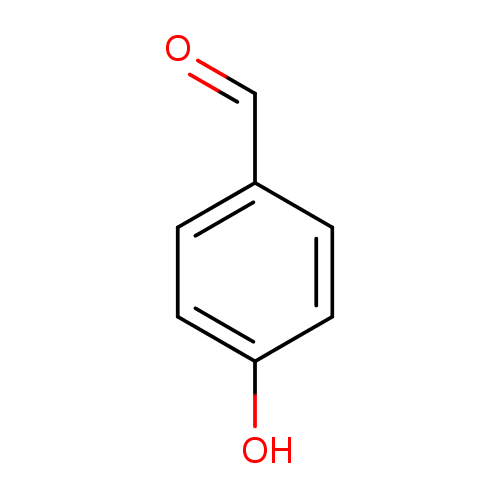
4-hydroxybenzaldehyde |
|---|
| Description: | A hydroxybenzaldehyde that is benzaldehyde substituted with a hydroxy group at position C-4. |
|---|
|
Structure |
|
|---|
| Synonyms: | |
|---|
|
Chemical Formula: |
C7H6O2
|
|---|
| Average Molecular Weight: |
122.12 |
|---|
| Monoisotopic Molecular
Weight: |
122.0367794368 |
|---|
| InChI Key: |
RGHHSNMVTDWUBI-UHFFFAOYSA-N |
|---|
| InChI: |
InChI=1S/C7H6O2/c8-5-6-1-3-7(9)4-2-6/h1-5,9H |
|---|
| CAS
number: |
123-08-0 |
|---|
| IUPAC Name: | 4-hydroxybenzaldehyde |
|---|
|
Traditional IUPAC Name: |
P-hydroxybenzaldehyde |
|---|
| SMILES: | [CH](C1(C=CC(O)=CC=1))=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as hydroxybenzaldehydes. These are organic aromatic compounds containing a benzene ring carrying an aldehyde group and a hydroxyl group. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic oxygen compounds |
|---|
| Sub Class | Organooxygen compounds |
|---|
|
Direct Parent |
Hydroxybenzaldehydes |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Hydroxybenzaldehyde
- Benzoyl
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Benzenoid
- Monocyclic benzene moiety
- Organic oxide
- Hydrocarbon derivative
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework |
Aromatic homomonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
117 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 117 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 8.45 mg/mL at 25 °C | Not Available | | LogP | 1.35 | HANSCH,C ET AL. (1995) |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0900000000-298ab6a83ac989405983 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-1900000000-12ad1640340af91bc8e8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0036-9000000000-ddc48e682b255bd53c5e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0900000000-785058db9c1fbc1b5950 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0900000000-c22365c838c065b99ed8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0100-9300000000-491fc6287b2479ddc1a6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0900000000-298ab6a83ac989405983 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-1900000000-12ad1640340af91bc8e8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0036-9000000000-ddc48e682b255bd53c5e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0900000000-785058db9c1fbc1b5950 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0900000000-c22365c838c065b99ed8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0100-9300000000-491fc6287b2479ddc1a6 | View in MoNA |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-00di-9500000000-2c1739a8a2bc288e4a5c | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Wu MC, Peng CF, Chen IS, Tsai IL (2011)Antitubercular chromones and flavonoids from Pisonia aculeata. Journal of natural products 74, Pubmed: 21542597
- Roux A, Xu Y, Heilier JF, Olivier MF, Ezan E, Tabet JC, Junot C (2012)Annotation of the human adult urinary metabolome and metabolite identification using ultra high performance liquid chromatography coupled to a linear quadrupole ion trap-Orbitrap mass spectrometer. Analytical chemistry 84, Pubmed: 22770225
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|