|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110165 |
|---|
|
Identification |
|---|
| Name: |
isopentenyl diphosphate |
|---|
| Description: | Trianion of isopentenyl diphosphate arising from deprotonation of the three OH groups of the diphosphate. |
|---|
|
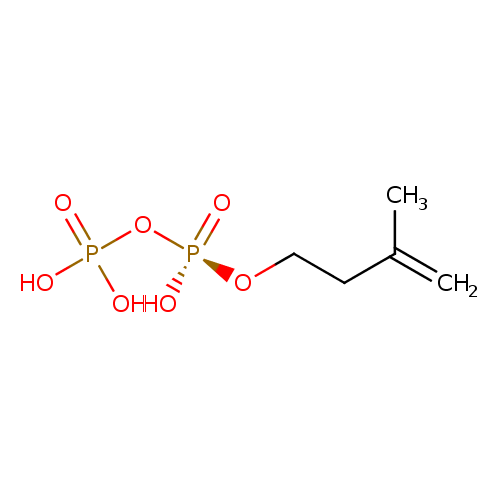
Structure |
|
|---|
| Synonyms: | -
isopentenyl-pp
-
isopentenyl pyrophosphate
-
IPP
-
δ(3)-isopentenyl-PP
-
Δ3-isopentenyl-PP
-
3-methylbut-3-enyl diphosphate
-
3-methylbut-3-enyl pyrophosphate
|
|---|
|
Chemical Formula: |
C5H9O7P2
|
|---|
| Average Molecular Weight: |
243.07 |
|---|
| Monoisotopic Molecular
Weight: |
246.0058257599 |
|---|
| InChI Key: |
NUHSROFQTUXZQQ-UHFFFAOYSA-K |
|---|
| InChI: |
InChI=1S/C5H12O7P2/c1-5(2)3-4-11-14(9,10)12-13(6,7)8/h1,3-4H2,2H3,(H,9,10)(H2,6,7,8)/p-3 |
|---|
| CAS
number: |
358-71-4 |
|---|
| IUPAC Name: | 3-methylbut-3-en-1-yl diphosphate |
|---|
|
Traditional IUPAC Name: |
isopentenyl-diphosphate |
|---|
| SMILES: | C=C(C)CCOP([O-])(=O)OP([O-])(=O)[O-] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as isoprenoid phosphates. These are prenol lipids containing a phosphate group linked to an isoprene (2-methylbuta-1,3-diene) unit. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Lipids and lipid-like molecules |
|---|
| Sub Class | Prenol lipids |
|---|
|
Direct Parent |
Isoprenoid phosphates |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Organic pyrophosphate
- Isoprenoid phosphate
- Monoalkyl phosphate
- Alkyl phosphate
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -3 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Reichenberg A, Hintz M, Kletschek Y, Kuhl T, Haug C, Engel R, Moll J, Ostrovsky DN, Jomaa H, Eberl M (2003)Replacing the pyrophosphate group of HMB-PP by a diphosphonate function abrogates Its potential to activate human gammadelta T cells but does not lead to competitive antagonism. Bioorganic & medicinal chemistry letters 13, Pubmed: 12657258
- Nagaki M, Kannari H, Ishibashi J, Maki Y, Nishino T, Ogura K, Koyama T (1998)Substrate specificity of thermostable farnesyl diphosphate synthase with alkyl group homologs of isopentenyl diphosphate. Bioorganic & medicinal chemistry letters 8, Pubmed: 9873578
|
|---|
| Synthesis Reference: |
Kao, Chai-Lin; Kittleman, William; Zhang, Hua; Seto, Haruo; Liu, Hung-Wen. Stereochemical Analysis of Isopentenyl Diphosphate Isomerase Type II from Staphylococcus aureus Using Chemically Synthesized (S)- and (R)-[2-2H]Isopentenyl Diphosphates.Organic Let |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|